1-7Department of Pharmaceutical Analysis and Quality Assurance, DKSS`s Institute of
Pharmaceutical Science and Research (For Girls),Swami-Chincholi, Bhigwan ,
(Affiliated to Dr.Babasaheb Ambedkar Technological University, Lonere,Pune),Maharashtra, India.
8Department of Pharmacology, Dattakala College of Pharmacy, Bhigwan,
(Affiliated to Savitribai Phule Pune University, Pune), Maharashtra, India.
Corresponding author email: chinababu.rao@gmail.com
Article Publishing History
Received: 25/09/2025
Accepted After Revision: 10/12/2025
The colorimetric determinations of Paracetamol in the bulk and pharmaceutical dosage form were carried out with different colouring reagents. The production of a coloured solution served as the foundation for the techniques. The qualitative or quantitative determination is based on the colour formation reactions of Paracetamol with colouring reagents. The wavelengths (ʎmax) were used 430,450,468,505,528,606,635,640,650 and 715nm. The calibration curves were constructed from the concentrations ranging from 0.1- 100 µg/mL and regression coefficient values were found to be >0.998. The % RSD values of the precision were found to be < 2. The methods were more accurate and values were found in between 99-101%. The methods were shown good robustness values and % RSD values were found to be <2.
Paracetamol, Colorimetric method, Colouring reagents
Chinababu D, Babar V. B, Yogita S. M, Sonali P. K, Nikita Y. M, Shital S. R, Harshala S. S, Aleesha S.A Review on Colorimetric Determinations of Paracetamol by Spectrophotometry. International Journal of Biomedical Research Science (IJBRS). 2025;01(3).
Chinababu D, Babar V. B, Yogita S. M, Sonali P. K, Nikita Y. M, Shital S. R, Harshala S. S, Aleesha S. A Review on Colorimetric Determinations of Paracetamol by Spectrophotometry. International Journal of Biomedical Research Science (IJBRS). 2025;01(3). Available from: <a href=”https://shorturl.at/S8dp2“>https://shorturl.at/S8dp2</a>
INTRODUCTION
Paracetamol (figure-1) is an analgesic and antipyretic [1]. Numerous spectrophotometric techniques have been documented for determining paracetamol,formed the red dye with diazotized 4-nitroaniline (DNAN) in sodium carbonate medium1, majority of published methods were on hydrolysis of the compounds leading to the Schiff base formed with a substituted benzaldehyde2, reaction of sodium nitrite with drug forms the diazonium salt and coupling reagent was 1-Napthol3, 2,4,6-trimethoxybenzaldehyde coupling formed pink colour2, the drug forms the colour with p-dimethyl amino benzaldehyde in the 95% ethanol and 2M HCl5, estimation of drug in plasma with sodium nitrite, one of the most often used Cl reagents is luminol [6]. Several oxidizing chemicals, including permanganate (MnO4-2), oxidize it, hydrogen peroxide (H2O2), periodate (IO4-2), and hypochlorite (ClO–).
Luminol undergoes oxidation to yield 3-aminophthalate, an excited state product that emits blue light with a maximum wavelength of 425 nm. Additionally, diperiodatocuprate (III) (DPC), diperidoatonicklate (IV) (DPN), and diperidodatoargentate (III) (DPA)are among the other high and unusual oxidation state transition metal complexes that are being used more and more for analytical purposes7. Microwave assisted hydrolysis of paracetamol with 2,2-(1,4-phenylenedivinylene) bis-8-hydroxyquinoline8, the colorimetric reagents Fe+3 and K3[Fe(CN)6] were employed for the determination of compound with exhaled breath condensate technique (EBC), Fe+3 reduced to Fe+2 and K3[Fe(CN)6] chelated the Fe+2 9 , oxidative coupling reaction of para amino phenol with p-xylenol (2,5-diethylphenol)10, reduce the compound with cerric ammonium sulphate11, the process produces a prussian blue (PB) complex by oxidizing paracetamol with Fe(III) and then reacting with ferricyanide in the presence of HCl12 , charge transfer complex formed between chromogenic reagent of chlorferone with drug13, oxidative coupling reaction of paracetamol with phenylephrene hydrochloride in ambient oxygen and in an alkaline media, produce a stable, water-soluble indophenol dye14, coupled reaction of 1-napthol15, indirect determination of paracetamol with Luminol-H2O2-Fe(CN)(-3)617 and based on the oxidation between drug and Fe(CN)(-3)6, it prevents reaction between luminol and hydrogen peroxide [18].
The analyte oxidised with K3[Fe(CN)6] at ambient temperature in ammonical aqueous solution by using anionic ion exchange column in the flow injection-spectrophotometric method19,20, talkaline hydrolysis of the analyte formed blue indophenol dye with 8-quinolinol in presence of KIO421-25, reaction with 2-nitroso-1-naphthol-4-sulfonic acid26, Stable colour complex produced with a Folin-Ciocalteau reagent27, formation of 2-nitro-5-acetamidophenol derivative with nitrous acid in an alkaline medium28.intrusion of salicylates and salicylamide in the colorimetric nitration method and grieves the estimation of paracetamol29,based on ring-nitration of the drug30, using chlorferone31, using ZrO2 (IV) and NH4NO3 (V)32 ,Colorimetric method of Glynn and Kendal 33, reduction of tris(2,2′-bipyridyl) ruthenium(III)34, luminol-permanganate based reaction35, using N, N-dibromo dimethylhydantoin36, 4-(Dimethylamino) benzaldehyde react with N-(4-hydroxyphenyl) ethanamide in 2M HCl after heating37, luorophore heroin reagent was used with NaClO oxidizing agent and pH was optimized at 10 with borate buffer38. NaClO oxidizes the analyte and excess of the oxidant determined with o-tolidine dichloride as chromogenic reagent at 430 nm 39.Paracetamol was dissolved in 4M sulphuric acid and treated with 10.0 mg of sodium bismuthate in the presence of 1M HCl and 1M acetic Acid. It exhibited a stable bluish-violet colour40.
Paracetamol to p-aminophenol that reacts with S2- in the presence of Fe3+ as oxidant to create a dye that resembles methylene blue and has an ʎmax at 540 nm41.The N-(4-hydroxyphenyl) ethanamide on the hydrolysis formed p-aminophenol and oxidant is dissolved oxygen in the alkaline medium, further formed in to N-acetyl-p-benzoquinone imine, which is capable to produce green indophenol dye with tiron42, estimate the drug by using cadmium pentacyanonitrosylferrate modified glassy carbon electrod43, Fe+3 ions oxidize paracetamol and salicylamide when 1,10-phenantroline is present44.
MATERIAL AND METHODS
The materials were used in the colorimetric methods paracetamol, diazitized 4-nitroaniline1,HCl, benzaldehyde, Na2CO3, 2,4,6-trimethoxybenzaldehyde2, resorcinol, ammonium sulfamate3, sodiumnitrite, 1-Naphthol3,4,6, 4-amino phenol, Na2SO4, tetrahydroxycalix, indophenol dye4, dimethylamino benzaldehyde5, chloroacetic acid, NaOH, sulphamic acid,ethanol, luminol, KMnO4, H2O2, KOH7, diperidodatoargentate (III)7, K3[Fe(CN)6], FeCl3.6H2O9, sodium periodate, tylenol10, ascarbic acid, phenylephrine hydrochloride, 2,6-dichloro indophenol, n-polopiryna11, styrene, maleic anhydride, benzoyl peroxide, tetrahydro furan, diethyl ether, acetonitrile, poly (styrene-alt-maleic acid), methanol12, 2,2-(1,4-phenylene divinylene) bis-8-hydroxyquinoline, ferric chloride, p-xylenol (2,5-diethylphenol), potassium ferricyanide12,16, protryliptyline HCl, chlorferone13,14,31, cerric amonium sulphate13, Iron (III)15, Zirconium (IV) oxide, ammonium trioxovanadate (V)32, N,N-Dibromo dimethylhydantoin36, O-tolidine39, Cadmium Pentacyanonitrosylferrate43
Preparation of sample solutions: The majority of published methods were reported the preparation sample solutions depends on acid hydrolysis of the paracetamol with substituted benzaldehyde forms the Schiff base2, microwave assisted hydrolysis4, few methods have been reported on coupling reactions with coupling reagents2, oxidation of the drug with oxidizing agents and oxidation state transition metal complexes and formation of charge transfer complexes7.
RESULTS AND DISCUSSION
The created procedures were validated in accordance with several recommendations, and the results produced fell within the acceptable range. The reported methods were shown good linearity and regression coefficient values were found to be greater than 0.999. The linearity range were found to be 0.5-20 µg/mL1 for batch method 1-150 µg/mL for FI method1, 20-100 µg/mL2, 2-10 µg/mL3, 1.5-12 µg/mL for conventional UV method for cloud point extraction technique the linearity range reduced to 0.14 – 1.5 µg/mL4 (figure-2), 1-5 mg/mL5, 25-400 mg/L6, 0.075-0.75 mg/L7, 0.44-5.5 mg/L8, 0.2-10 µg/mL9, 25-600 µg/mL10, 0.01-0.5µg/mL12, 40-160 µg/mL13, 50-500 mg/L28, 0.5-24 µg/mL14, 2-10µg/mL15, 0.1- 2.4µg/mL16, 2-16 mg/mL17. The %RSD of the accuracy obtained results were found to be within the limits as per guidelines.
The concentration ranges of the reported methods were found to be from 0-60 mg/ml, 0.8 – 3.36 mL, 0 to 10 µg/ml, 0.01 – 0.70, 1.5—12 mg/mL, 0.075-0.75 mg/L, 0.2–10.0 μg/mL, 2.5-25 µg/mL and >70 µg/mL , 25 to 60 mg/ml,0.25-30 ppm19, 0.2-20 ppm20, f 0.01-0.5 mg/mL, 10.00–60.00 g/mL, 0.5-24 µg/mL, 2 – 10 μg/mL, 1.0-3.0 mL. The reported values were found the good LOD & the lowest LOD is 0.0369 µg/mL whereas the highest LOD is 40.0 mg/mL. The various methods have been reported and values were found to be 40.0 mg/mL, 0.0007 mg/mL, 0.2 µg/mL, 0.49 µg/mL, 0.007 µg/mL, 0.0578 µg/mL, 0.02 g/mL, 0.03 g/mL, 0.0448 mg/mL. The quantification limit values were found to be 0.002 mg/L, 0.166 mg/mL, 0.1753 g/mL, 0. 10 g/mL, 0.08 mg/mL, 0.149 mg/mL, 0.121mg/mL. The %RSD values of robustness were found to be within the limits as per guidelines.
The review article focused on the colourimetric determination of paracetamol with different colouring reagents and validation parameters. The Hayati filik et al4 reported lowest linearity concentration range from 0.14-1.5 µg/mL with CPE method by using with colourant calixarene (CAL4) in the presence of KIO4 oxidant and colour of the solution was blue . Cloud point phase separation was done with Triton X-114 (surfactant micelles) by addition of Na2SO4. It is the high sensitive method because of detection limit was found to be 40ng/mL.4
Figure 2: Absorption spectrum of the reaction product with and without Triton-X1144
Ca=ʎmax-580nm ; Cb= 590nm
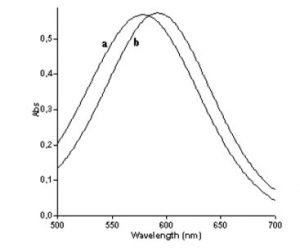
Paracetamol estimated in urine by using different colouring reagents and their linearity
concentration values were reported in table 1.
Table 1. Colorimetric estimation of Paracetamol in Urine4
| Method | Linearity range
(µg/mL) |
LOD
(ng/mL) |
Reference |
| Phenol | 100-800 | NR | Welch et al47 |
| o-Cresol | 0.3-12 | 100 | Criado et al45 |
| Xylenol | 20-400 | NR | Chen et al49 |
| Resorcinol | 2-100 | 900 | Bocxlaer et al48 |
| 8-hydroxyquinoline | 0-2.5 | NR | Morris et al50 |
| Derivative UV | 5-30 | 2000 | Parojicic et al51 |
| Diazotisation | 2-10 | NR | Heirwegh et al46 |
| ELSD | 1-100 | 300 | Criado et al52 |
| UV-Visible | 1.5-12 | 500 | Hayati Filik et al4 |
| CPE-UV-Visible | 0.14-1.5 | 40 | Hayati Filik et al4 |
CONCLUSION
The paracetamol was estimated colorimetrically by using spectrophotometer with different coloring reagents. The charge transfer complex formed between chromogenic reagent of chlorferone with drug and shown sensitivity of the method. Different oxidation reactions were carried with oxidizing (coupling) agents were used with alkaline or acidic medium and produces the more intense colour, determined in visible range. The colourant calixarene (CAL4) in the presence of KIO4 oxidant the drug was produced blue colour. This reagent used for the estimation of drug in the urine, with cloud point phase separation Triton X-114 (surfactant micelles) was used by addition of Na2SO4.
It is the high sensitive method because of LOD was found to be 40 ng/mL and linearity range was found to be 0.14-1.5 µg/mL. The drug produces the stable colored complex with Folin-Ciocalteau reagent it enhance the sensitivity and stability of the method. The other colorants were used benzoyl peroxidepoly (styrene-alt-maleic acid), 2,2-(1,4-phenylene divinylene) bis-8-hydroxyquinoline, ferric chloride, P-xylenol (2,5-diethylphenol), potassium ferricyanide12,16, protryliptyline HCl, chlorferone13,14,31, cerric amonium sulphate13, Iron (III)15, Zirconium (IV) oxide, ammonium trioxovanadate (V)32, N,N-Dibromo dimethylhydantoin36, O-tolidine39, Cadmium Pentacyanonitrosylferrate43.
Conflict of Interest: The authors have stated that they don’t have any conflicting interests.
ACKNOWLEDGEMENTS
The authors express gratitude to Dattakala Shikshna Santhan’s Management for providing requirements.
REFERENCES
- Mouayed Q. Al-Abachi.; Raghad Sinan.; Zaineb Falah. Batch and Flow-Injection Spectrophotometric Methods for Determination of Paracetamol in Pharmaceutical Preparations by Coupling with Diazotized 4-Nitroaniline. Iraqi Journal of Science., 2008, 49(2),11-20.
- Haruna Baba.; Nkiruka P.; Egbucheand Cyril O Usifoh. Spectrophotometric determination of paracetamol in syrup formulations using 2,4,6-trimethoxybenzaldehyde as a coupling agent. Jour of Chem and Pharm Res., 2013, 5(12),957-960.
- Buddha Ratna Shrestha.; Raja Ram Pradhananga. Spectrophotometric Method for the Determination of Paracetamol. Jour of Nepal Chem Socie., 2009, 24, 39-44.
- Hayati Filik.; Izzet Sener.; Sema Demirci Ceki; Emine KiLiC.; and Resat Apak. Spectrophotometric Determination of Paracetamol in Urine with Tetrahydroxycalix[4] arene as a Coupling Reagent and Preconcentration with Triton X-114 Using Cloud Point Extraction. Chem Pharm Bull., 2006,54(6), 891—896.
- Usifoh C O.; Adelusi SA.; and Adebambo RF. Colorimetric Determination of Paracetamol in Raw Material and in Pharmaceutical Dosage Forms. Pak J Sci Ind Res.,2002, 45 (1),7-9.
- Fathima Shihana.; Dhammika Menike Dissanayake.; Paul Ivor Dargan.; and Andrew Hamilton Dawson. A Modified Low Cost Colourimetric Method for Paracetamol (Acetaminophen) Measurement in Plasma. Clin Toxicol (Phila)., 2010, 48(1), 42–46.
- Abdul Hakeem.;Naqeebullah Khan.; Rehana Kamal.; Attiq-Ur-Rehman Kakar.; Samiullah.; Habiba Taj.; Khair Un Nisa. Flow Injection Determination of Paracetamol in Pharmaceutical Formulations Using Luminol –Diperiodatoargentate (III) System with Chemiluminescence Detection. Journal of Population Therapeutics and Clinical Pharmacology.,2024, 31(5),1578-1590.
- Hayati Filika.; Mustafa Hayvalib.; Emine Kilicb. Sequential Spectrophotometric determination of Paracetamol and p-aminophenol with 2,2-(1,4-phenylenedivinylene)bis-8-hydroxyquinoline as a novel coupling reagent after microwave assisted hydrolysis. Analytica Chimica Acta., 2005, 535,177-182.
- Fariba Pourkarim.;Elaheh Rahimpour.; Maryam Khoubnasabjafari.; Vahid Jouyban‑Gharamaleki.; Afshin Gharakhani.; Abolghasem Jouyban. Validation of a colorimetric method for determination of paracetamol in exhaled breath condensate. Chemical Papers., 2021,75, 2901–2906.
- Jalil Tavakoli Afshari.; Tsan-Zon Liu. Rapid spectrophotometric method for the quantitation of acetaminophen in serum. Analytica Chimica Acta., 2001,443,165–169.
- Izabela Muszalska.; Marianna Zajac.; Gregorz Wrobel.;Maria Nogowska. Redox methods validation of paracetamol and ascorbic acid in pharmaceutical preparations. Acta Poloniae Pharmaceutica-Drug Research., 2000,57 (1),27-32.
- Mohseni,N.; Bahram, M.; Farhadi, KH.; Naja-Moghaddam, P.; Keshvari, F.; Spectrophotometric determination of paracetamol using hydrogel based semi solid-liquid dispersive microextraction. Scientia Iranica.,2014, 21(3),693-703.
- Kumble Divya.; Badiadka Narayana.; Majal Sapnakumari. Sensitive Spectrophotometric Determinations of Paracetamol and Protriptyline HCl Using 3-Chloro-7-hydroxy-4-methyl-2H-chromen-2-one. Hindawi.,2013,1-6.
- Firas Muhsen Al-Esawati.; and Raeed Megeed Qadir. Spectophotometric determination of paracetamol via oxidative coupling with phenylephrine hydrochloride in pharmaceutical preparations. J Duhok Univ (Pure and Eng. Sciences)., 2011, 14(1),52-57.
- Raymond T Iorhemen.; Aondona M. Iorhemba.; Royalty Sambo.;Winifred U. Nande. Spectrophotometric determination of paracetamol in drug formulations with 1– naphthol. Int Jour of Adv Chem., 2017,5(2),86-90.
- Nagendra,P. Spectrophotometric estimation of paracetamol in bulk and pharmaceutical formulations. E-Journal of Chemistry., 2011, 8(1),149-152.
- Eesa M. Thalj Aljuboury. Spectrophotomitric Determination of Paracetamol by using eitherVaniline or KMnO4 oxidizing agents. Tikrit Jour of Pharm Sci., 2017, 12(2),111-119.
- Alapant, A G.; Zamora L L.; Calatayud, J M. Indirect determination of paracetamol in pharmaceutical formulations by inhibition of the system luminal–H2O2–Fe(CN)3 (6)– chemiluminescence. Pharm. Biomed. Anal., 1999, 21(2),311-317.
- Martinez Calatayud J.; Pascual Marti M.C.; Sagrado Vives, S. Determination of paracetamol by a flow injection spectrophotometric method. Analytical Letters.,1986, 19(19-20),2023-2038.
- Martinez Calatayud J.; Sagrado Vives, S. An oxidative column for the flow injection analysis-spectrophotometric determination of paracetamol. Jour of Pharm and Biomed Ana.,1989, 7(10),1165-1172.
- Bouhsain, Z.; Garrigues, S.; Moralesrubio, A.; Delaguardia, M. Flow injection spectrophotometric determination of paracetamol in pharmaceuticals by means of on-line microwave-assisted hydrolysis and reaction with 8- hydroxyquinoline (8-quinolinol), Chim. Acta.,1996, 330(1),59-69.
- Inamdar, M C.; Gore, M A.; Bhide, R V. Estimation of Paracetamol by colorimetric method. Ind J Pharm.,1974,36,7-9.
- Davis, D R.; Fogg, DT.; Burns, D.; Wragg, J S. A colorimetric method for determination of phenacetin and Paracetamol. Analyst.,1974,99,12-18.
- Ellcock, CT.; Fogg, Selective colorimetric determination of paracetamot by means of an indophenol reaction. Analyst.,1975,100(1186),16-8.
- Murfin, JW.; Wragg, JS. A colorimetric method for the determination of phenacetin and paracetamol. II. A manual procedure for the determination of phenacetin or paracetamol in formulations. Analyst.,1972,97(157),670-67
- Shihabi, ZK.; David, Colorimetric assay for acetaminophen in serum. Ther Drug Monit.,1984,6,449–453.
- Gupta, RN.; Pickersgill, R.; Stefanec, Colorimetric determination of acetaminophen. Clin Biochem.,1983,16,220–221.
- Hale PW Jr, Poklis A. Evaluation of a modified colorimetric assay for the determination of acetaminophen in serum. J Anal Toxicol.,1983, 7(5),249-2
- Archer, CT.; Richardson, An improved colorimetric method for the determination of plasma paracetamol. Ann Clin Biochem.,1980, 17,45–46.
- Bailey, Colorimetry of serum acetaminophen (paracetamol) in uremia. Clin Chem., 1982,28,187–90.
- Divya, K.; Narayana, B.; Sapnakumari, M. Sensitive spectrophotometric determinations of paracetamol and protriptyline HCl using 3-chloro-7-hydroxy-4-methyl-2H-chromen-2-one. Int Sch Res Notices.,2013,1-6.
- Ihemekwa, O.C.; Ukoha, P.O.; Ekere,N.R. Spectrophotometric determination of paracetamol using zirconium (IV) oxide and ammonium trioxovanadate (V). J.Chem.Soc. Nigeria. 2016., 41(2), 46-51.
- Shihana, F.; Dissanayake, D.; Dargan, P.; Dawson, A. A modifed low-cost colorimetric method for paracetamol (acetaminophen) measurement in plasma. Clin Toxicol. 2010., 48(1),42–46.
- Ruengsitagoon,;Liawruangrath,S.;Townshend, A. Flow injection chemiluminescence determination of paracetamol. Talanta, 2006., 69(4), 976–983.
- Easwaramoorthy, D.; Yu, Y.; Huang,H. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Analytica Chimica Acta, 2001., 439,95.
- Kumar, KG; Letha, R. Determination of paracetamol in pure form and in dosage forms using N, N-dibromo dimethylhydantoin. Jour of Pharma and Biomed Anal, 1997.,15, 1725–1728.
- Usifoh, CO.; Adelusi, SA.; Adebamco, RF. Colorimetric determination of paracetamol in raw material and in pharmaceutical dosage forms. Pak Jour of Sci and Ind Res,2002., 45(1), 7 – 9.
- Vilchez, JL.; Blanc, R.; Avidad, R.; & Navalo, A. Spectrofluorometric determination of paracetamol in pharmaceuticals and biological fluids. Journal of Pharmaceutical and Biomedical Analysis,1995.,13(9),1119 – 1125.
- Fatibello-Filho, O.; Vieira, HJ. Spectrophotometric flow injection procedure to indirect determination of paracetamol in pharmaceutical formulations using o-toulidine as reagent. Ecletica Quimica, 2008, 33(2), 47 – 54.
- Pavan Kumar, GVSR.; Bhuvan Kumar, G.; Chandra Sekhar, T.; Murthy, BS. Spectrophotometric Determination of Paracetamol Using Sodium bismuthate as Chromogen. Int Jour of Res in Chem and Env,2012., 2(1), 231-235.
- Xu, C.; & Li, B.; Spectrophotometric determination of paracetamol with microwave assisted alkaline hydrolysis. Spectrochimica Acta. Part A. Molecular and Biomolecular Spectroscopy, 2004., 60,186.
- Cekic, SD.; Filik, H.; Apak, R. Simultaneous spectrophotometric determination of paracetamol and p-aminophenol in pharmaceutical products with tiron using dissolved oxygen as oxidant. Journal Analytical Chemistry, 2005., 60(11),1019-1023.
- Razmi, H.; and Harasi, M. Rapid and accurate amperometric determination of acetaminophen in pharmaceutical preparations and spiked human blood serum samples at cadmium pentacyanonitrosylferrate modified glassy carbon e J. Iran. Chem. Soc, 2008.,5(2), 296-305.
- Abbas, A.; Nahid, S.; and Ali, R.Z. Spectrophotometric determination of salicylamide and paracetamol in biological samples and pharmaceutical formulations by A differential Kinetic Method. Acta Chim.Slov, 2006.,53, 357–362.
- Andres Criado.; Soledad Cardenas.; Mercedes Gallego.; and Miguel Valcarcel. Fast urinary screening for paracetamol using on-line microwave assisted hydrolysis and spectrophotometric detection. The Analyst,2000.,125,1179-1183.
- Heirwegh, P. M.; Fevery, J. Clin. Chem,1967.,13, 215-219.
- Welch,M.; Conney, A.H. A simple method for the quantitative determination of N-Acetyl-p-aminophenol. Clinical. Chemistry,1965., 11(12),1064-1067.
- Van Bocxlaer,F.; Clauwaert, K.M.; Lambert,W.E.;De Leenheer, A.P.Clin.Chem, 1997.,43,627-634.
- Chen, CF.; Tseng, YT.; Tseng, HK.; Liu, Automated spectrophotometric assay for urine para aminophenol by an oxidative coupling reaction. Ann Clin Lab Sci, 2004.,34(3):336-40.
- Morris, HC.; Overton, PD.; Ramsay, JR.; Campbell, RS.; Hammond, PM.; Atkinson, T.; Price, Development and validation of an automated enzyme assay for paracetamol (acetaminophen). Clin Chim Acta. 1990.,187(2),95-104.
- Parojc,ICJ.; Karljikovic-Rajic,; Duric, Z.; Jovanovic, M.; Ibric,S. Biopharm. Drug Dispos, 2003., 24, 309-314.
- Criado, A.; Cardenas, S.; Gallego, M.; Valcarcel, M. Usefulness of the evaporative light scattering detector for direct screening of biological fluids. Anal. Chim. Acta, 2001.,435, 281-288.



