1Department of Pharmacology, Dr. Ravi Patil College of Pharmacy, Belagavi. India
2-3Department. Pharmacology, Shree Devi College of Pharmacy, Mangalore, India
*Corresponding Author Email: sanjiv.karale@gmail.com
Article Publishing History
Received: 06/06/2025
Accepted After Revision: 25/07/2025
The present study was aimed to explore the ameliorative effect of Emilia sonchifolia plant extract (ESPE) on cisplatin (CIS) induced nephrotoxicity in experimental rats. The animals were divided into 4 groups of 6 animals each. Normal (saline 10 ml/kg p.o) for 9 days, cisplatin (8mg/kg i.p on the 5th day of treatment), test group of low dose (ESPE 250 mg/kg p.o), test group of high dose (ESPE 500 mg/kg p.o) for 9 days in combination with cisplatin (on 5th day of treatment). After completion of 9 days dosing with test drug, on 10th day (after 6 days from CIS administration), the experimental rats were anesthetized by ether inhalation blood samples were collected from each rat by intra cardiac puncture and used for the estimation of biomarkers (creatine, urea and uric acid).
Kidney of each animal was isolated, one part used for evaluation of antioxidants (GSH, SOD and catalase) and the other for histopathology. ESPE explored a significant (P<0.01) protection against CIS-induced nephrotoxicity by suppressing serum creatinine, urea and uric acid and by elevating the antioxidant parameters GSH, SOD and catalase at different doses compared to CIS treated group. Thus, investigational finding suggests that ESPE possess potential benefits against nephrotoxicity induced by anti-cancer drug CIS.
Cisplatin, Emilia Sonchifolia, Renal protective, Cytotoxic drug, Oxidative stress, Creatinine.
Sanjiv K,* Theertha T, Jagadish V. K. Ameliorative Effect of Emilia Sonchifolia Plant Extract on Cisplatin Induced Renal Injury in Albino Wistar Rats. International Journal of Biomedical Research Science (IJBRS). 2025;01(2).
Sanjiv K,* Theertha T, Jagadish V. K. Ameliorative Effect of Emilia Sonchifolia Plant Extract on Cisplatin
Induced Renal Injury in Albino Wistar Rats. International Journal of Biomedical Research Science (IJBRS). 2025;01(2). Available from: <a href=”https://shorturl.at/s74Zv“>https://shorturl.at/s74Zv</a>
INTRODUCTION
Nephrotoxicity is an intrinsic adverse effect of certain anticancer drugs. Anticancer drugs have a narrow therapeutic index and therapeutic dose of such drugs usually produces significant nephrotoxicity. Cisplatin (CIS), cis-diamine dichloro platinum (II), is one of the most powerful antineoplastic agents used to treat a wide range of human malignancies such as testicular, ovarian, bladder, head and neck, esophageal, small cell lung cancer, cervical, bladder and other genito-urinary tumours. The toxic effects of cisplatin in kidney affects approximately 25–35% of patients treated with a single dose, thereby limiting its use in higher doses and compromising its chemotherapeutic efficacy. CIS treatment is hampered by significant side effects such as neurotoxicity, nephrotoxicity, myelosuppression, ototoxicity, nausea and vomiting. The most serious and usually dose limiting toxicity of cisplatin is renal and it is associated with its metabolism [1-3].
CIS -induced nephrotoxicity is also closely associated with an increase in lipid peroxidation in the kidney tissues. This antitumoral drug also causes generation of reactive oxygen species (ROS), such as superoxide anion and hydroxyl radical that deplete the GSH (reduced glutathione) levels and inhibit the activity of antioxidant enzymes in renal tissue. This ROS may produce cellular injury and necrosis via several mechanisms including peroxidation of membrane lipids, protein denaturation and DNA damage [4]. In allopathy till date there is no effective drug reported to treat the nephrotoxicity of cisplatin. Several studies have focused currently on traditional herbal medicines to evaluate novel therapeutic drugs for acute kidney injury (AKI) therapy.
Various herbal medicines, including pomegranate, Prosthechea michoacana, Zingiber officinale, red ginseng etc. have protective effects against cisplatin-induced acute kidney injury with vivo experiment. Emilia sonchifolia is a soft annual herb which grows up to 40 cm in height, leaves are simple, lyrate-pinnate with large terminal lobe, flowers purplish with corymbose heads. Fruits are oblong containing many seeds. The plant occurs in open field and waste lands. The plant is effective in treatment of fever, tonsillitis. The juice is used for eye infections. The herb is useful in conditions like cough, bronchial disorder, piles, worm infections, diarrhea, swelling and diabetics. The other reported activities of the plant include anti-anxiety, hepatoprotective, anti-cataract and anti-convulsant activities. Tribal uses the juice of crushed whole plant for wound healing [5,6].
MATERIALS AND METHODS
Animals: Albino Wistar rats of either sex weighing 180-250g were procured from animal house of Shree Devi College of pharmacy, Mangalore and housed at 25° ± 5°C, relative humidity 50 ± 5% in a well-ventilated animal house under 12:12 h light dark cycle. All the rats were provided with commercially available standard pellet diet, water and libitum. All the studies conducted were approved by Institutional Animal Ethical Committee (IAEC), Shree Devi College of Pharmacy, Mangalore (SDCP/IAEC/15/2020) according to prescribed guidelines of committee for the purpose of Control and Supervision of Experiments in Animals (CPCSEA), Government of India.
Chemicals and reagents: Cisplatin was purchased from Sigma Aldrich, Bangalore and biochemical kits from Robonik India Pvt Ltd in Mumbai. Other chemicals and reagents used were analytical grade and were procured from standard companies.
Preparation of Emilia Sonchifolia Plant Extract (ESPE): The plant, E. Sonchifolia was collected from Kerala, India. E. sonchifolia whole plants were washed with distilled water immediately after collection. The plants were chopped into small pieces, air dried at room temperature (26 ± 1) °C for about 10 days and ground into powder (grinder purchased locally) to store in an airtight container. Plant powder was soaked in n- hexane solvent and kept in the shaker for 48 hours at room temperature. The extract was collected and concentrated at 40°C under reduced pressure using a rotary evaporator. The dried extract was stored at 4°C until further use. The remaining residue was extracted again with the fresh solvent to ensure complete extraction and performed phytochemical analysis.7-8
Experimental Design: The present study was designed for 10 days. After acclimatization for one week, the male rats were randomly categorized into four equal groups in separate polypropylene cages with six rats each. Group I: Normal (saline 10 ml/kg p.o) for 9 days Group II: CIS (8mg/kg i.p) on the 5th day of treatment. Group III: Low dose (250 mg/kg, p.o) ESPE + CIS on the 5th day of treatment. Group IV: High dose (500 mg/kg, p.o) ESPE + CIS on the 5th day of treatment. After completion of 9 days dosing with test drug, on 10th day, the experimental rats were anesthetized by ether inhalation and blood was collected from each rat by intracardiac puncture for the estimation of biomarkers (creatine, urea and uric acid). Then animals were sacrificed and kidneys were isolated, one part used for evaluation of antioxidants (GSH, SOD and catalase) and the other for histopathology [9-10].
Assessment of renal protective activity of ESPE – Evaluation of serum markers and endogenous antioxidant parameters: The concentration of blood markers such urea, uric acid, and creatinine in Heparinized serum samples were measured with semi-auto analyser (Robonik) and commercial kits.
Each rat’s kidney half was homogenized in a solution of saline (0.05M, pH 7.4) and ice-cold 10% trichloroacetic acid phosphate buffer. For fifteen minutes, the renal homogenates were centrifuged at 15,000 rpm. The generated supernatants were utilized in colorimetric measurements of GSH, SOD, and catalase. The GSH content of the kidney tissue was assessed using Ellmann’s technique. Aebi approach was used to evaluate the activity of the catalase enzyme. Additionally, the Kakkar et al. approach was used to evaluate the SOD activity in renal tissues [11-13].
Histopathological study: Specimens of kidney tissues of each group were fixed in 10% of buffered formalin and processed with paraffin wax. For histopathological examination, 5 micrometer sections were stained with hematoxylin and eosin (H&E) for the examination using light microscope [14].
Data and Statistical analysis: The data obtained by the various parameters was statistically evaluated by one-way analysis of variance (ANOVA) followed by Tukey-Karmer multiple comparison tests using Graph Pad Prism software. The definitive results as expressed in mean ± standard error of mean and p< 0.05 was considered to be statistically significant [15-16].
RESULTS
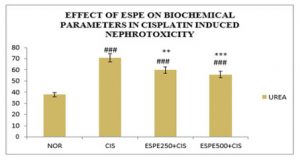
Effect of ESPE on renal function biomarkers: A significant (P<0.001) protection was explored on serum markers in ESPE pre-treated animals against CIS -induced disruption on renal system (Table 1 & Fig 1,2). A significant (P<0.001) rise in serum creatinine, urea and uric acid content was exhibited in CIS alone treated group compared to the normal group. The pre-treatment with ESPE at both doses (250 mg/kg and 500 mg/kg) groups showed a significant (P<0.01 & P<0.001) depletion in the level creatinine and urea compared to CIS alone treated groups. And The pre-treatment with ESPE (250 mg/kg and 500 mg/kg) groups explored a significant (P<0.05 & P<0.01) decline in the level of Uric acid compared to CIS alone treated groups.
Table 1. Effect of ESPE on serum biomarkers in CIS-induced nephrotoxicity
| TREATMENT | CREATININE (mg/dl) | UREA (mg/ml) | URIC ACID (mg/ml) |
| NORMAL | 0.958±0.009 | 37.858±0.032 | 1.587±0.021 |
| CIS | 4.457±0.019### | 70.808±0.048### | 6.312±0.029### |
| ESPE 250+CIS | 3.362±0.176**### | 59.785±2.643**### | 4.936±0.416*### |
| ESPE 500+CIS | 2.087±0.373***## | 55.761±3.063***### | 4.229±0.548**### |
n=6, values are expressed in Mean ±SEM, one‑way ANOVA followed by Tukey‑Kramer multiple comparison test. *p<0.05, **P<0.01, ***P<0.001 when compared to cisplatin ##P<0.01, ###P<0.001 when compared to normal.
Figure 1: Effect of ESPE on serum creatinine and uric acid in CIS induced nephrotoxicity
n=6, values are expressed in Mean ±SEM, one‑way ANOVA followed by Tukey‑Kramer multiple comparison test. *p<0.05, **P<0.01, ***P<0.001 when compared to cisplatin ##P<0.01, ###P<0.001 when compared to normal.
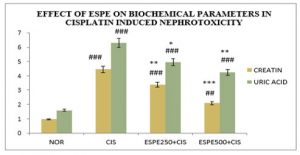
Figure 2: Effect of ESPE on serum urea in CIS induced nephrotoxicity
n=6, values are expressed in Mean ±SEM, one‑way ANOVA followed by Tukey‑Kramer multiple comparison test. **P<0.01, ***P<0.001 when compared to cisplatin ###P<0.001 when compared to normal.
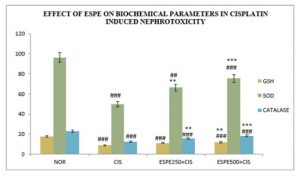
ESPE ameliorates the CIS ‑mediated decrease in antioxidant defense of renal system: The pre-treatment with ESPE revealed beneficial role on endogenous antioxidants against CIS -induced nephrotoxicity (Table 2 & Fig 3). A significant (P<0.001) decline in GSH was observed in CIS alone treated groups compared to normal groups. The pre-treated with ESPE (250 mg/kg and 500 mg/kg) animals exhibit a significant (P<0.05 & P<0.01) elevation in GSH compared to CIS alone treated groups. There was significant (P<0.001) fall in SOD and catalase was found in CIS alone treated animals compared to normal rats. The pre-treated with ESPE (250 mg/kg and 500 mg/kg) groups were explored significant (P<0.01 & P<0.001) rise in SOD and catalase compared to CIS alone treated animals.
Table 2. Effect of ESPE on antioxidants in CIS induced nephrotoxicity.
|
TREATMENT |
GSH
(ηM/g wet gland) |
SOD
(U/mg wet gland) |
Catalase
(U/mg wet gland) |
| NORMAL | 17.509±0.058 | 96.262±0.007 | 22.75±0.012 |
| CIS | 8.737±0.006### | 49.566±0.007### | 12.08±0.017### |
| ESPE250+CIS | 11.006±0.615*### | 66.332±2.649**### | 15.607±0.726**### |
| ESPE500+CIS | 11.777±0.950**### | 75.380±5.974***## | 18.052±1.202***### |
n=6, values are expressed in Mean ±SEM, one‑way ANOVA followed by Tukey‑Kramer multiple comparison test. *p<0.05, **P<0.01, ***P<0.001 when compared to cisplatin ##P<0.01, ###P<0.001 when compared to normal.
Figure 3: Effect of ESPE on antioxidants in CIS induced nephrotoxicity in rats.
n=6, values are expressed in Mean ±SEM, one‑way ANOVA followed by Tukey‑Kramer multiple comparison test. *p<0.05, **P<0.01, ***P<0.001 when compared to cisplatin ##P<0.01, ###P<0.001 when compared to normal.
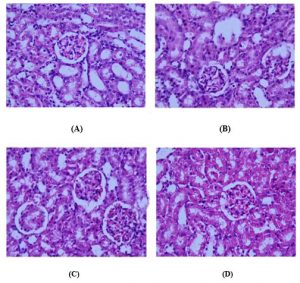
Histological Analysis: The renal cortex of normal rats revealed a normal corpuscular and tubular histological structure. In CIS-treated rats, degenerative changes were noticeably observed in renal tissues. These changes were in the form of luminal dilatation with excessive cast accumulation; the renal corpuscles showed dilated capsular space with condensed and even degenerated glomerulus. Inflammatory cells infiltration and vascular congestion were noticed within the renal cortex and pyknotic nuclei were present in renal tubules. The kidneys of rats treated with low dose ESPE (250 mg/kg) + CIS and high dose ESPE (500mg/kg) + CIS revealed less histological damage in renal corpuscles and renal tubules. Mild tubular degeneration with luminal dilatation and inflammatory cell infiltration were seen within the renal cortex. (Fig 4).
Figure 4: Histopathological slides of Rat kidney tissue in CIS induced nephrotoxicity
Normal kidney section with normal renal corpuscle and renal tubules. Rats treated with CIS with congested shrank and completely degenerated glomeruli, debris in the lumen of some renal tubules, and pyknotic nuclei in renal tubules and (D) Treatment with ESPE 250 mg/kg and 500 mg/kg normal renal corpuscle and renal tubule more or less like normal structure.
DISCUSSION
The present study was aimed to evaluate the protective effect of ESPE on renal toxicity induced by CIS in wistar rat and the study revealed highly significant protective effects of ESPE against CIS.
CIS is widely used to treat a variety of tumors, such as the head and neck region, testis, liver, prostate, ovary, lung, and cervix. Renal damage, the most common deleterious effect limiting cisplatin medicines, affects around 30% of individuals. According to certain research, ROS reactions play a crucial role in mediating CIS-induced renal toxicity. Herbs and plants are naturally occurring resources with a long history of therapeutic use.
The human search for a potent nephroprotective agent has led people to seek out appropriate sources, such as the traditional medical system that has less adverse benefits. The antioxidant-rich plants Rubia cordifolia, Hybanthus enneaspermus, Pongamia pinnata, Echina pallidum, Ginkgo biloba, and Nigella sativa shown a notable defense against nephrotoxicity caused by cisplatin. The current study also sought to reduce nephrotoxicity caused by cisplatin therapy by using ethanolic extracts of ESPE concurrently with treatment [17,1].
In CIS treated group induced renal toxicity, there is significant (P<0.001) elevation of urea, creatinine and uric acid when compared with normal rats. Serum creatinine and urea are termed as the major indicators or of nephrotoxicity and hepatorenal syndrome. Serum creatinine is the endogenous substance of muscle metabolism and eliminated unchanged by the renal system. The increase in creatinine level in the blood may be due to the abnormality of glomerular filtration process in the kidney. Urea is the waste substance generated during protein metabolism of by Urea cycle by the liver.
The deficiency of GFR and a decrease in blood volume leads to increase in urea level in serum. Uric acid is by-product produced during purine biotransformation by the enzyme urate oxidase (uridase). The increase in uric acid level might be due to kidney urate elimination and inefficiency of glomerular filtration in nephrons. Further, it may lead to hyperuricemia due to more tubular reabsorption, mediated by urate exchanger and voltage sensitive urate channel in proximal tubules [18-19]. The pre-treatment with ESPE successfully amend the increased levels of creatinine, urea and uric acid induced by CIS.
Oxidative stress damage and development of reactive oxygen species (ROS) were suggested in the pathogenesis of CIS and other cytotoxic drugs induced nephrotoxicity. Cellular injury causes due to disruption of dynamic equilibrium between pro-oxidant and free radicals scavenge by antioxidants. In present study, CIS administration leads significant decline in GSH level. The depletion in GSH content in renal tissues after CIS therapy is due to the toxic metabolite acrolein. Acrolein binds GSH in the plasma membrane and disrupts antioxidant defense system and increase the ROS and results in the necrosis of the tubular cells of kidney. In the current work, a significant decline in SOD and catalase level was observed after CIS administration.
SOD and catalase are the vital antioxidant enzymes which converts oxygen molecules into non-toxic products. Increase in ROS leads to decrease in SOD level. Elevation in the level H2O2 is due to the inhibition of catalase action. The depletion of catalase in turn also prevents the SOD action. The decline in these antioxidant enzymes is mainly due to more ROS and lipid peroxidation [20-22]. The pretreatment with ESPE lower and higher doses significantly (P<0.01) increases the level GSH, SOD and catalase after CIS treatment.
The findings of serum markers and antioxidant parameters were correlated with histopathological examination. The CIS therapy exhibit pathological abnormality such as decrease in the size of Bowman’s capsule, congestion and dilation of blood vessels, inflammatory infiltration [23]. The pre-treatment with ESPE at both doses successfully augmented the toxic effects induced by CIS renal system by exploring beneficial effects such as, no change in the size of Bowman’s capsule, slight inflammation and no congestion of blood capillaries.
CONCLUSION
The present investigation reveals the potent renal protective activity of ESPE (250 mg/kg and 500 mg/kg, p.o) against cisplatin induced renal injury in experimental rats. The prophylactic treatment and efficacy of herb could be attributed to its potential antioxidant property and free radical scavenging activity, modulation in serum markers and protection of histopathological features. Further research is required to establish the fact clinically.
ACKNOWLEDGEMENTS
The authors are thankful to the Principal and Management of Shree Devi College of Pharmacy, Mangalore, for providing all facilities and their immense support for our work.
Conflicts of Interest: There is no Conflict of Interest.
REFERENCES
- Spandana U et al. Nephroprotector activity of hydro alcoholic extract of Tinospora cordifolia roots on cisplatin induced nephrotoxicity in rats. Drug Invention Today 2013; 5:281-7.
- Mohamed AK, Safaa BG and Mohamed OM. The effect of some natural antioxidants against cisplatin-induced neurotoxicity in rats: behavioral testing. Heliyon 2020; 6:1-7.
- Rupal P, Rohit S and Renu B. Protective role of Emblica officinalis hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats. Toxicology Reports 2018; 5:270-7
- Divyasree S and Cherupally KN. Amelioration of cisplatin-induced nephrotoxicity by extracts of Hemidesmus indicus and Acorus calamus. Pharmaceut Biol 2010;48(3): 290–5
- Muhammad OI et al. Nephroprotective Effects of Alhagi camelorum against Cisplatin-Induced Nephrotoxicity in Albino Wistar Rats. Molecules 2022;27(941):1-16.
- Smitharani et al. Investigation on the wound healing activity of aqueous extract of Emilia sonchifolia (L.) Dc. Int J Herbal Medicine 2017; 5(6): 34-9.
- Rahman MA, Akter N, Rashid H, Ahmed NU, Uddin N, Islam MS. Analgesic and anti-inflammatory effect of whole Ageratum conyzoides and Emilia sonchifolia alcoholic extracts in animal models. Afr J Pharmacy and Pharmacol. 2012 May 29;6(20) PP1469-76.
- Dominic S et al. Effect of Emilia sonchifolia (Linn.) DC on alcohol- induced oxidative stress in pancreas of male albino rats. Asian Pacific J Tropical Medicine 2011; 973-7.
- Zhao Y, Dai W. Effect of phloretin treatment ameliorated the cisplatin-induced nephrotoxicity and oxidative stress in experimental rats. Pharmacognosy Magazine. 2020 Apr 1;16(69):207.
- Zahra E et al. Ameliorative Effects of Gallic Acid on Cisplatin-Induced Nephrotoxicity in Rat Variations of Biochemistry, Histopathology, and Gene Expression. BioMed Research International 2021;1-11.
- Ellmann GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82:70-7.
- Aebi H. Catalase. In: HU Bergmeyer. Ed. Methods in Enzymatic Analysis. Vol. 3. Academic Press: New York; 1983. p. 276–86.
- Kakkar PS, Das B, Viswanathan PNA. Modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984; 21:130–2.
- Alhoshani AR et al. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC nephrology 2017 Dec;18(1):1-0.
- Smitha J and Sreedevi A. Effect of the Ethanolic Extract of Scoparia dulcis in Cisplatin induced Nephrotoxicity in wistar rats. Indian J Pharmaceut Edu Res 2015;49(4):S68-S74.
- Hardevinder Pal S, Thakur Gurjeet S and Randhir S. Evaluation of the renoprotective effect of syringic acid against nephrotoxicity induced by cisplatin in rats. J Applied Pharmaceutical Sci 2021; 11(1):80-5.
- Ehsan N et al. Mitigation of cisplatin induced nephrotoxicity by casticin in male albino rats. Brazilian J Biol 2023;83:1-9.
- Tayyaba A et al. Acacia hydaspica R. Parker ethyl‑acetate extract abrogates cisplatin‑induced nephrotoxicity by targeting ROS and inflammatory cytokines. Scientifc Reports 2021;11(17248):1-16.
- Sonam S, Priya D, K Sairam and Alakh NS. Amelioration of cisplatin induced nephrotoxicity by Phylanodiflora (L.) Greene. Indian J Exp Biol 2020;58:691-8.
- Zhu W, Wansen S, Xi S, Ye W and Meilan Z. Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Expr 2020; 10(58):1-11.
- Sonam S, Apurva J and Siva H. Protective Effect of Withania coagulans Fruit Extract on Cisplatin-induced Nephrotoxicity in Rats. Phcog Res 2017;9(4):354-61.
- Sardar H, Komal KP and Guruvayoorappan C. Emilia Sonchifolia-A Critical and Comprehensive Review of its Diverse Medicinal Potential and Future as Therapeutic. Pharmacogn J 2023;15(6):1143-9.
- Doa I, Abdulsalam H, Mohammed AA and Amani S. Ameliorative Effect of Olea europaea Leaf Extract on Cisplatin Induced Nephrotoxicity in the Rat Model 2023:1-13.



