1Ağrı İbrahim Çeçen University, Faculty of Pharmacy, Ağrı, Turkey
2Ağrı İbrahim Çeçen University Faculty of Pharmacy / Department of Basic
Pharmaceutical Sciences / Division of Basic Pharmacy Sciences 04100 Ağrı, Türkiye
Corresponding author Email: hycelik@agri.edu.tr
Article Publishing History
Received: 12/01/2025
Accepted After Revision: 09/03/2025
Since ancient times, many plant species have been used to treat diseases, and this practice continues today. In recent years, interest in natural and herbal treatments has increased in both developed and developing countries. Traditional herbal medicines have become a preferred choice for disease treatment due to their greater availability and lower cost compared to synthetic products. For this purpose, compounds with various biological and pharmacological properties have been isolated from many different plants. Among these herbal sources, quercetin stands out as a highly popular compound.
The name “quercetin” is derived from the Latin word “Quercetum,” meaning “oak forest.” It is a flavonoid compound abundantly found in plants, especially in vegetables and fruits. Extensive studies have demonstrated that quercetin exhibits a wide range of biological activities, including antioxidant, antibacterial, antimicrobial, antiviral, antifungal, anti-inflammatory, antihypertensive, anticancer, antiobesity effects, and protection against testicular damage. Recent developments have confirmed its safety; however, the lack of clear understanding of its mechanism of action limits its clinical application. Therefore, further scientific investigations are necessary to elucidate the mechanisms and address the unresolved aspects of this important compound.
Quercetin, Biological activity, Herbal medicines.
Gizem. G, Celik H. Biological Activity of Quercetin. International Journal of Biomedical Research Science (IJBRS). 2025;01(1).
Gizem. G, Celik H. Biological Activity of Quercetin. International Journal of Biomedical Research Science (IJBRS). 2025;01(1). Available from: <a href=”https://shorturl.at/C9UeF“>https://shorturl.at/C9UeF</a>
INTRODUCTION
Quercetin is derived from the Latin word “Quercetum,” meaning “oak forest.” It belongs to one of the six subcategories of flavonoid compounds and is commonly found in higher plants as a glycoside, primarily in the forms of isoquercetin, rutin, and hyperin [1]. The compound was first isolated and identified by Szent-Györgyi in 1935 [2]. Quercetin is present in various fruits such as apples, plums, mangoes, blueberries, cranberries, red grapes, and green leafy vegetables, as well as in many seeds, buckwheat, nuts, olive oil, honey, beans, lettuce, onions, broccoli, coriander, dill, and green tea. Onions are considered the most abundant source of quercetin and are widely used both as a food and medicinal plant [3].
Quercetin supplementation may prevent many chronic diseases due to its antioxidant, anti-inflammatory, immunoprotective, anticarcinogenic, and antidiabetic activities. Additionally, it influences lipid peroxidation, inhibits platelet aggregation, and modulates capillary permeability and mitochondrial biogenesis. Due to its high solubility and bioavailability, quercetin is increasingly being incorporated into novel preparations aimed at improving human health [4].
Onions (Allium cepa L.), known for their high quercetin content (Figure 1), are among the oldest and most widely cultivated crops worldwide. They have been extensively used both as a vegetable and for medicinal purposes. The most common varieties are purple, white, and yellow onions [5].
Figure 1.Allium cepa L. (Onion) plant

Two primary chemical groups are present in the onion structure: flavonoids and alkenylcysteine sulfoxides. Anthocyanins, a subgroup of flavonoids, are responsible for the red-purple coloration in certain onion types, while flavonols such as quercetin and its derivatives contribute to the yellow and brown hues. Alkenylcysteine sulfates are the compounds that give onions their characteristic pungent odor and taste. Among approximately 20 flavonols identified in onion species, two quercetin derivatives—quercetin-3,4-O-diglucoside and quercetin-4-O-monoglucoside—account for 80–85% of the total flavonoid content. In onions, quercetin is found in three forms: the aglycone and two glucosides (quercetin 4‐O‐glucoside and quercetin‐3,4‐O‐diglucoside)[5].
Garlic (Allium sativum L.), a member of the Alliaceae family (Figure 2), is a cultivated plant typically growing 25–100 cm tall. It features greenish-white or pink flowers and consists of herbaceous roots, stems, leaves, cloves, and flower parts. Native to the steppes of Central and Western Asia, garlic is now cultivated worldwide.
Beyond its culinary use, garlic has been valued for its medicinal properties since the Middle Ages. Historically, it was used to combat epidemics; for instance, during World War II, Russian soldiers applied crushed garlic to wounds to prevent infections. Garlic can be consumed raw or in the form of pills, capsules, and extracts. It is generally considered safe when used in moderate amounts, although excessive consumption may cause stomach irritation [6].
Figure 2. Allium sativum (Garlic) plant

Buckwheat (Fagopyrum esculentum) (Figure 3) was historically neglected in Western countries during the 20th century due to competition from wheat, despite being a popular food during the 17th and 19th centuries. Buckwheat is native to Central Asia and later spread to regions with cold climates. Its ability to grow under harsh climatic conditions and its rich nutritional profile have led to increased use in both food and traditional medicine over time [7].
Four flavonol glycosides have been identified in buckwheat extracts, including rutin, quercetin, kaempferol-3-rutinoside, and a small amount of flavonol tri-glycoside. Rutin, the primary flavonoid in buckwheat, is a quercetin aglycone bound to the disaccharide rutinose. Buckwheat is unique among pseudo-cereals as a significant source of rutin; indeed, studies have not found rutin as a dietary source in any other grain or pseudo-grain besides buckwheat [8].
Figure 3. Buckwheat (Fagopyrum esculentum)

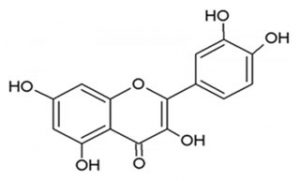
IUPAC Name: 3,3′,4′,5,6-Pentahydroxyflavone
Molecular Formula: C₁₅H₁₀O₇ (Figure 4)
Molecular Weight: 302.24 g/mol
Density: 1.799 g/cm³
Appearance: Pure crystalline powder
Melting Point: 316 °C (601 °F, 589 K)
Storage Conditions: Store at room temperature, protected from sunlight
Solubility: Nearly insoluble in water; soluble in alkaline solutions [9].
Figure 4. Chemical structure of quercetin
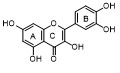
Derivatives of Quercetin: Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromene-4-one) [10], an important flavonoid, contains five hydroxyl groups located at positions 3, 5, 7, 3′, and 4′. Some of these hydroxyl groups undergo glycosylation to form various quercetin glycosides, which represent the main quercetin derivatives.
Structurally, quercetin-3-O-glucoside (isoquercetin) contains a glucose moiety bound to quercetin. Similarly, the binding of galactose to the 3-OH position produces hyperoside (quercetin 3-O-galactoside). The addition of a rhamnosyl group to either the 3-OH or 7-OH positions leads to the synthesis of quercetin 3-O-rhamnoside and quercetin 7-O-rhamnoside, respectively. Some quercetin derivatives also contain disaccharides such as rutinose, which consists of rhamnose and glucose linked as α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose. The attachment of this disaccharide to the 3-OH position results in rutin, an important quercetin derivative. Other derivatives include quercetin with arabinofuranose bound at the 3-OH position. Some quercetin glycosides contain more than two sugar residues; examples include enzymatically modified isoquercetin (EMIQ), which contains up to 10 glucose residues attached to the 3-OH of quercetin, and oligoglycosylated rutin, with up to five additional glucose residues attached to rutin’s glucose moiety.
Methylated quercetin derivatives also exist. For example, tamarixetin (quercetin 4′-methyl ether) has a methyl group at the 4′ position. Rhamnetin (7-O-methylquercetin) contains a methyl group at the 7-OH position. Dimethylated quercetin, known as rhamnazin, possesses methyl groups at both the 3′- and 7-OH positions. Isorhamnetin, also called 3-methylquercetin or rhamnetol, is another methylated flavonol. It can be glycosylated to form isorhamnetin 3-O-rutinoside (narcissin), isorhamnetin 3-O-rutinoside-7-O-glucoside, and isorhamnetin 3-O-rutinoside-4′-O-glucoside. There is significant structural diversity in quercetin derivatives, combining both glycosylation and methylation at various hydroxyl groups. For example, tamarixetin 3-O-β-D-glucoside contains a methyl group at the 4′ position and a glucose moiety at the 3′ position (Table 1) [11].
Table 2. Derivatives of Quercetin.
 |
Modifications of quercetin in | ||
| Selected Quercetin Derivatives | Ring A | Ring B | Ring C |
| Quercetin-3-O-glucoside(isoquercetrin) | _ | _ | 3-OH to 3-O-glucoside |
| Quercetin-3-O-galactoside(hyperoside) | _ | _ | 3-OH to 3-O-galactoside |
| Quercetin-3-O-rhamnoside(Quercitrin) | _ | _ | 3-OH to 3-O-rhamnoside |
| Quercetin-7-O-glucoside | 7-OH to
7-O-glucoside |
_ | _ |
| Quercetin-3-O-rutinoside | _ | _ | 3-OH to 3-O-rutinoside |
| Quercetin-3-methyl ether(Isorhamnetin) | _ | _ | 3-OH to 3-O-methyl ether |
| Quercetin 3,3′-dimethyl ether | _ | 3′-OH to
3′-O-methyl ether |
_ |
| Quercetin-4′-glucoside
Spiracoside |
_ | 4′-OH to
4′-O-glucoside |
_ |
Bioavailability and Pharmacokinetics of Quercetin
Initial research on the pharmacokinetics of quercetin in humans indicated that its oral bioavailability is very low, with only about ~2% absorbed after a single oral dose. The estimated absorption rate of quercetin glucosides—the naturally occurring form of quercetin—ranged from 3% to 17% in healthy individuals who received a 100 mg dose [12]. Quercetin and its derivatives have been found to be stable in gastric acid; however, there is no clear evidence regarding their absorption in the stomach. Studies suggest that quercetin is primarily absorbed in the upper segment of the small intestine [13].
Among the derivatives of quercetin, the conjugated glycoside forms have been shown to exhibit better absorption than quercetin aglycone. Purified quercetin glycosides have the ability to interact with sodium-dependent glucose transporters in the intestinal mucosa and may therefore be absorbed in the small intestine in vivo. The absorption efficiency of quercetin glycosides can vary depending on the type of sugar moiety attached [14]. Current evidence suggests that quercetin glycosides—particularly those found in onions and shallots—are absorbed more efficiently than rutinosides, which are the predominant quercetin glycosides in tea. These glycosides are effectively hydrolyzed in the small intestine by β-glucosidases into aglycone forms, which are then readily absorbed. Additionally, glucuronic acid and sulfuric acid conjugates of quercetin have demonstrated superior absorption compared to the aglycone [15].
The absorption of quercetin is further influenced by factors such as the specific glycosylation pattern, the food matrix in which quercetin is consumed, and the co-administration of dietary components such as fiber and fat [16]. Therefore, the type of sugar and its conjugation site significantly affect quercetin absorption. Once absorbed, quercetin undergoes metabolism in various organs including the small intestine, colon, liver, and kidneys. Metabolites formed by biotransformation enzymes in the small intestine and liver include methylated, sulfated, and glucuronidated forms. A distribution study conducted in rats and pigs revealed that the highest concentrations of quercetin and its metabolites were found in the lungs (rats) and in the liver and kidneys (pigs) [17].
Quercetin and its derivatives have been shown to be metabolized into various phenolic acid derivatives by intestinal microbiota and by enzymes present in the epithelial cells of the intestinal mucosa. These metabolites may then undergo further absorption, conversion, or excretion. Additionally, microbial ring fission of the aglycone structure occurs in both the small intestine and the colon, leading to the breakdown of quercetin’s backbone and the formation of smaller phenolic compounds [18].
Plasma and liver analyses have shown that the concentration of quercetin derivatives is lower in the liver than in the plasma, with hepatic metabolites being predominantly methylated (90–95%) [19]. Limited studies suggest that quercetin undergoes methylation, sulfation, and glucuronidation in the liver [20]. Continuous dietary intake of quercetin has been found to result in its accumulation in the blood, significantly increasing its plasma concentration in correlation with the dietary dose [21]. In human blood, quercetin is primarily present in conjugated forms, mainly as glycosides. Conjugated metabolites such as isorhamnetin (3′-methylquercetin) and sulfated glycosides account for approximately 91.5% of total quercetin metabolites, while minor metabolites include glucuronides and methylated derivatives [22].
Boulton further reported that the fractionation of quercetin reduced its binding to plasma proteins—99.4% of which is albumin—thus potentially enhancing its cellular bioavailability [23]. Limited research suggests that quercetin and its metabolites tend to accumulate in organs involved in its metabolism and excretion, and mitochondria may serve as potential intracellular sites of quercetin accumulation [24]. The kidney has been reported to be an important excretory organ. In humans, the concentration of quercetin in urine increases with both the dose and the duration of intake, particularly after consuming quercetin-rich juice. Benzoic acid derivatives have been proposed as common metabolites of quercetin [25].
Human subjects may absorb significant amounts of quercetin from foods or dietary supplements, and its elimination is relatively slow. The reported elimination half-life ranges from 11 to 28 hours, although some studies have also cited a mean terminal half-life of approximately 3.5 hours [26,27]. Total recovery of carbon-labeled quercetin (^14C-quercetin) in urine, feces, and exhaled air has been found to vary considerably between individuals (Ay et al., 2008) [28].
Further studies suggest that glycosylated quercetin (quercetin glycosides) is absorbed more efficiently than its aglycone form. Moreover, the co-administration of quercetin with vitamin C, folate, and other flavonoids has been reported to enhance its bioavailability [24]. Additionally, quercetin absorbed in high amounts has been shown to be extensively metabolized and ultimately eliminated through the lungs [29].
All these studies indicate that quercetin glycosides are absorbed in the upper segment of the small intestine, where they undergo methylation by biotransformation enzymes, followed by sulfo-substitution and glucuronidation in both the small intestine and liver. Ultimately, these metabolites are excreted via the kidneys in the urine.
Antimicrobial Activity of Quercetin: The antimicrobial mechanisms of action of various phytochemicals are currently being extensively investigated to enhance their potential in drug development. Among them, quercetin has been demonstrated to be a promising natural antimicrobial agent effective against a wide range of pathogenic microorganisms [30].
Quercetin exhibits antibacterial activity against multiple bacterial strains, particularly those affecting the gastrointestinal, respiratory, urinary, and intestinal tracts [31]. Its antibacterial efficacy is attributed to its solubility and its interaction with bacterial cell membranes. It has shown effectiveness against both Gram-positive and Gram-negative bacteria; however, Gram-negative bacteria tend to be more resistant to the bactericidal effects of quercetin compared to Gram-positive bacteria [32]. This difference in resistance is likely due to variations in the composition and structure of the bacterial cell membranes.
Some derivatives of quercetin have demonstrated greater antibacterial activity against Gram-negative bacteria than against Gram-positive ones. Factors influencing this include the solubility of quercetin, as well as the phosphorylation and sulfation of its hydroxyl groups, which may alter its antimicrobial potential [33].
Studies have reported varying minimum inhibitory concentrations (MIC) for quercetin’s antimicrobial effects. Notably, quercetin has exhibited a synergistic effect when combined with certain chemotherapeutic agents and antibiotics, enhancing bacterial growth inhibition [34, 35].
When Pseudomonas fluorescens, a bacterium commonly associated with food spoilage, was treated with quercetin in combination with lactoferrin and hydroxyapatite, the minimum inhibitory concentration (MIC) was significantly reduced compared to treatment with quercetin alone [34]. Furthermore, quercetin demonstrated notable antibacterial efficacy when used in combination with other antibiotics against methicillin-resistant Staphylococcus aureus (MRSA), indicating its potential as an adjunct therapeutic agent to combat antibiotic-resistant bacterial strains [36].
Anticancer Activity of Quercetin: Quercetin has been recognized as a potential natural anticancer agent due to its wide range of biological activities, including antioxidant, anti-inflammatory, antiproliferative, proapoptotic, and antiangiogenic effects. These properties have prompted increasing interest in its application for cancer treatment. Studies have demonstrated that quercetin, whether used alone or in combination with other agents, can exert significant anticancer effects by inducing cell death in cancer cells. However, there is also evidence indicating that quercetin may exhibit toxic and genotoxic effects under certain conditions. For example, in vivo studies in rats have shown that oral administration of quercetin at daily doses ranging from 0.2% to 0.5% can cause measurable toxic and genotoxic effects detectable in urine and fecal samples [37].
Nevertheless, a comprehensive evaluation of the existing studies suggests that quercetin is selectively cytotoxic to cancer cells while being less toxic or non-toxic to healthy cells, highlighting its promise as an alternative candidate for cancer therapy [10]. Furthermore, with the advancement of research and its widespread use, quercetin has been granted GRAS (Generally Recognized as Safe) status by the U.S. Food and Drug Administration (FDA) [30].
Extensive research in recent years has explored the therapeutic potential of quercetin, revealing a broad spectrum of biological activities beyond its well-known antioxidant properties. Quercetin, either alone or in combination with other compounds, has been shown to induce apoptosis in malignant cells (Chien et al., 2009) [38]. Interestingly, quercetin exhibits a dual role, acting as both an antioxidant and a prooxidant—it protects healthy cells while selectively targeting and eliminating cancerous cells [39]. Moreover, several studies have confirmed its efficacy against multidrug-resistant cancer types [40].
In vitro studies have demonstrated quercetin’s anticancer effects across various malignancies, including glioma, osteosarcoma, cervical cancer, prostate cancer, breast cancer, colorectal xenografts, myeloid leukemia, and oral cavity cancers [9].
Anti-Alzheimer’s Activity of Quercetin: Currently, no widely effective drug treatment exists to delay, slow, or cure the onset of Alzheimer’s disease; most approved therapies only provide temporary symptomatic relief. Moreover, many synthetic drugs with antioxidant, anti-inflammatory, hypoglycemic, or hypolipidemic effects often cause side effects that limit their clinical application. In this context, the use of natural compounds for the treatment of neurodegenerative diseases such as Alzheimer’s disease, and metabolic disorders like Type 2 Diabetes Mellitus, presents a promising alternative. These agents are often inexpensive, easily isolated from natural sources, and have well-documented mechanisms of action and safety profiles. Quercetin, a dietary phytochemical, has emerged as a significant candidate in this regard [41].
Quercetin has demonstrated therapeutic efficacy in Alzheimer’s disease by improving learning, memory, and cognitive performance [42]. A study by [43] showed that quercetin administration inhibited acetylcholinesterase (AchE) and secretase enzymes in vitro, thus preventing acetylcholine degradation and reducing amyloid-beta (Aβ) production. Furthermore, Sabogal-Guáqueta et al. (2015) [44] reported that quercetin administration reversed extracellular β-amyloidosis and reduced tauopathies in the hippocampus and amygdala, as well as astrogliosis and microgliosis. These findings suggest that quercetin may help preserve cognitive and emotional functions in transgenic mouse models of Alzheimer’s disease.
Wang et al. (2014) [45] investigated the effects of long-term quercetin administration on cognition and mitochondrial dysfunction in a mouse model of Alzheimer’s disease. They observed that quercetin improved mitochondrial dysfunction by restoring mitochondrial membrane potential, reducing reactive oxygen species (ROS) production, and restoring ATP synthesis. Additionally, quercetin increased the expression of AMP-activated protein kinase (AMPK), a key regulator of cellular energy metabolism. Activated AMPK may reduce ROS formation by inhibiting NADPH oxidase activity or by enhancing the antioxidant activity of enzymes such as superoxide dismutase-2 and protein degradation pathways. Moreover, AMPK activation decreased amyloid-beta (Aβ) accumulation, regulated amyloid precursor protein (APP) processing, and promoted Aβ clearance. These mechanisms likely explain some of the therapeutic benefits of quercetin on cognitive function and the reduction of Aβ-induced neurotoxicity. Furthermore, quercetin and its glycoside rutin have been reported to act as memory enhancers in scopolamine-induced memory impairment models in zebrafish, possibly by increasing cholinergic neurotransmission [46].
Antihypertensive Activity of Quercetin: Studies have shown that quercetin plays a role in preventing cardiovascular diseases and exhibits antihypertensive effects (Hollman et al., 2010) [47]. However, evidence regarding its effects on endothelial function, atherosclerosis, and insulin resistance remains insufficient [48]. The antihypertensive effect of quercetin in humans appears to be independent of the origin of hypertension, renin-angiotensin system status, oxidative stress, and other related factors (Rivera et al., 2008) [49]. The blood pressure-lowering effects of flavonols, including quercetin, have been demonstrated in several human studies (Hollman et al., 2010) [47]. For instance, a clinical trial by Edwards et al. (2007) [50] showed that quercetin significantly reduced both systolic and diastolic blood pressure in patients with stage 1 hypertension. Another study demonstrated that the systolic blood pressure-lowering effect of quercetin was significant in patients with the ApoE3 genotype, while no significant change was observed in those with the ApoE4 genotype among patients with metabolic syndrome. These findings suggest that although flavonols have antihypertensive effects, the response to quercetin may vary depending on the genetic background of the individual [51].
Oral ingestion of quercetin at doses ranging from 150 to 730 mg/day for four to ten weeks in humans has demonstrated antihypertensive effects. A randomized, double-blind, placebo-controlled, crossover study showed that daily intake of 730 mg quercetin for four weeks reduced both systolic and diastolic blood pressure in patients with stage 1 hypertension, but had no significant effect on those with prehypertension. In individuals with metabolic syndrome, intake of 150 mg quercetin per day for five weeks was reported to lower systolic blood pressure [52]. Furthermore, a double-blind, randomized clinical trial conducted on 72 women with type 2 diabetes found that daily intake of 500 mg quercetin for 10 weeks significantly reduced systolic blood pressure, although diastolic pressure was not significantly affected. Meta-analyses of several randomized controlled trials summarize that quercetin supplementation at doses greater than 500 mg/day for eight weeks significantly lowers both systolic and diastolic blood pressures [53].
Protective Activity Against Testicular Damage: Numerous studies over the past decade have confirmed that various congenital and pathological causes of testicular damage and impaired spermatogenesis contribute significantly to male infertility. Quercetin has been reported to play a protective role against testicular damage induced by diverse factors, including chemotherapeutic drugs, heavy metal exposure, environmental pollutants, and diabetes. Quercetin’s beneficial effects in diabetes-induced testicular damage are primarily attributed to its antioxidant, anti-apoptotic, and anti-inflammatory properties. For example, quercetin treatment at 20 mg/kg/day for six weeks alleviated diabetes-induced reductions in testicular total antioxidant capacity (TAC), superoxide dismutase (SOD), and catalase (CAT) levels, as well as the elevation of malondialdehyde (MDA), in Zucker Diabetic Fatty rats [54].
Anti-inflammatory and anti-allergic Activity: Quercetin, a flavonoid, has been confirmed as a potent and long-acting anti-inflammatory agent. Both animal and human studies have demonstrated its significant anti-inflammatory potential across various cell types [55]. Extracts containing quercetin have been utilized as key ingredients in several promising antiallergic drugs. Compared to cromolyn sodium (a known antiallergic drug), quercetin has shown a stronger ability to inhibit interleukin-8 (IL-8), as well as inhibiting interleukin-6 (IL-6) and increasing cytosolic calcium levels [56]. Its anti-inflammatory and anti-allergic properties have been validated in treating respiratory and food allergies [57].
Quercetin has repeatedly demonstrated anti-inflammatory effects on endothelial cells and monocyte/macrophage systems in vitro [58]. For instance, Li et al. [12] showed that quercetin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha (TNF-α) production in macrophages and lung A549 cells, and also suppresses LPS-induced IL-8 production in lung A549 cells. Furthermore, quercetin was shown to reduce LPS-induced TNF-α and interleukin-1 alpha (IL-1α) mRNA levels in glial cells, which also contributed to decreased neuronal cell death [59].
Quercetin may exert its anti-inflammatory effects by inhibiting enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX), which play key roles in inflammation [60]. Additionally, studies have indicated that quercetin modulates immune responses, potentially reducing post-exercise illness. Nieman et al. found that supplementation with quercetin and epigallocatechin-3-gallate (Q-EGCG) for two weeks in well-trained cyclists increased granulocyte counts and reduced inflammation after strenuous exercise [60]. Moreover, clinical studies have found that quercetin, along with resveratrol, EGCG, and genistein, can enhance both cellular and humoral immune functions [61].
Antioxidant Activity: Free radicals are naturally produced in the body during metabolism and are known to contribute to the development of many diseases. They can cause damage to cell membranes, induce gene mutations, accelerate aging, and lead to various conditions such as cardiovascular disease, liver damage, and diabetes. Hanasaki et al. (1994) [62] identified quercetin as the most effective free radical scavenger within the flavonoid family.
The chemical structure of quercetin reveals four hydroxyl groups in the benzodihydropyrane ring. This polyphenolic structure endows quercetin with strong antioxidant capacity, enabling it to eliminate free radicals produced in the body and help maintain cellular stability.
The in vitro antioxidant mechanisms of quercetin mainly include:
Direct scavenging of free radicals: Quercetin has demonstrated potent antioxidant activity, exhibiting the highest antioxidant effect among tested samples [63]. Additionally, Manca et al. [64] found that quercetin, when combined with liposomes and glycerol nanoparticles, effectively scavenged free radicals and protected human keratinocytes from hydrogen peroxide-induced damage in vitro.
Chelation of metal ions: Studies have shown that quercetin can chelate metal ions such as Cu²⁺ and Fe²⁺ through the catechol group in its structure, enhancing its antioxidant function. Tang et al. (2014) [65] modeled alcoholic liver disease by administering quercetin to adult male C57BL/6J mice and demonstrated that quercetin inhibited Fe²⁺-induced lipid peroxidation by binding Fe²⁺, thereby preventing iron overload and oxidative damage in alcoholic liver disease. Similarly, Babenkova et al. [66] showed through chemiluminescence studies that Fe²⁺ in dihydroquercetin-containing compounds becomes inactive, losing its ability to catalyze hydrogen peroxide decomposition and inhibit further hydroxyl radical formation. Thus, quercetin’s antioxidant role is partly attributed to its metal ion chelating properties.
Inhibition of lipid peroxidation: Inhibition of lipid peroxidation: Lim et al. [67] demonstrated that quercetin inhibits oxidative modification of low-density lipoprotein (LDL) by observing changes in the fluorescence intensity of thiobarbituric acid reactive substances, phosphatidylcholine hydroperoxides, and oxidized LDL. This inhibition prevents oxidative damage to LDL. Additionally, Mbikay et al. [68] confirmed that at low concentrations, quercetin increases LDL receptor (LDLR) expression, decreases PCSK9 secretion, stimulates LDL uptake, and thereby helps prevent oxidative damage to LDL.
Neuroprotective activity: Neurodegenerative disorders are typically late-onset, progressive, age-related brain diseases clinically characterized by reduced cognitive control, impaired motor activity, dyskinetic movements, and persistent changes in behavior and personality. The pathological features of disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease (HD) include the accumulation of mutant proteins—α-synuclein, amyloid-β (Aβ), and mutant Huntingtin (Htt), respectively—in the affected brain regions [69].
Aβ aggregation is a hallmark feature of Alzheimer’s disease [69]. Quercetin has demonstrated therapeutic efficacy in improving memory and learning in AD models [42]. Studies have shown that quercetin administration inhibits secretase and acetylcholinesterase (AChE) enzymes, thereby preventing acetylcholine degradation and reducing Aβ production [70]. Sabogal-Guaqueta et al. reported that quercetin administration reversed extracellular amyloidosis, reduced tauopathy, microgliosis, and astrogliosis in the amygdala and hippocampus, preserving cognitive and emotional functions in triple-transgenic AD mice [44].
Moreover, quercetin inhibits the formation of fibrillar Aβ proteins, likely due to its antioxidant properties that counteract cell lysis and the associated inflammatory cascade [71]. It has also been reported to reduce amyloid precursor protein (APP) maturation, thus altering Aβ synthesis and aggregation [72]. Several studies confirm that quercetin acts as an antioxidant, inhibits inducible nitric oxide synthase (iNOS), and modulates cyclooxygenase-2 (COX-2) expression, contributing to its anti-inflammatory effects. The glucuronidated, sulfated, and methylated metabolites of quercetin are well absorbed and also exhibit neuroprotective effects [73].
Sriraksa et al. assessed acetylcholinesterase levels as an indirect measure of cholinergic system activity and memory, finding that quercetin significantly reduced AChE levels in hippocampal neuron homogenates. This reduction increased acetylcholine concentration at synaptic terminals, improving cognitive outcomes in animals [74].
Parkinson’s disease (PD) is an age-related neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), with clinical symptoms including bradykinesia, tremor, and rigidity [75]. Cognitive impairment in PD has been strongly linked to cholinergic deficiency, and quercetin has been shown to significantly improve cognitive deficits induced by 6-hydroxydopamine (6-OHDA) administration [76].
Quercetin also protects against mitochondrial dysfunction and the progressive degeneration of dopaminergic neurons in transgenic mouse models of PD. In a study by Haleagrahara et al., oral administration of quercetin in the same model reduced striatal dopamine loss and decreased markers of oxidative stress, exerting neuroprotective effects [77]. Quercetin has been demonstrated to reduce the dose-dependent degradation of striatal dopamine [78], which was associated with a significant reduction in lipid peroxidation markers and a significant increase in striatal dopamine levels.
Oral administration of quercetin moderately but significantly attenuated striatal dopamine loss, behavioral disturbances, and nigrostriatal degeneration. The quercetin glycoside rutin was also tested in the 6-OHDA rat model, showing partial improvement in motor deficits. This effect correlated with a moderate but significant increase in striatal dopamine and brain glutathione (GSH) levels, along with reduced markers of lipid and protein oxidation. Zhang and colleagues investigated quercetin’s neuroprotective effects on PC12 cells and zebrafish models. They found that quercetin inhibited the overexpression of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) in PC12 cells and reduced the expression of pro-inflammatory genes such as IL-1β, COX-2, and TNF-α in zebrafish [79].
Huntington’s Disease (HD): The beneficial effects of quercetin on Huntington’s disease (HD) have been investigated. Studies examined pathological changes such as mitochondrial swelling, mitochondrial bioenergetics, oxidative stress, and neurobehavioral deficits following quercetin treatment. It was found that quercetin supplementation inhibited the respiratory chain reaction cascade, restored ATP levels, and reduced mitochondrial oxidative stress, as measured by lipid peroxidation [80].
Quercetin and Cardiovascular Protection: Quercetin exerts beneficial effects on cardiovascular diseases such as hypertension, atherosclerosis, ischemia-reperfusion injury, and cardiotoxicity [81]. These protective effects are closely related to the anti-inflammatory and antioxidant properties of quercetin. The cardiovascular protective mechanisms of quercetin include:
1.Reduction of systolic blood pressure, diastolic blood pressure, and mean arterial pressure.
- Decrease in lipid peroxidation, free fatty acids, phospholipids, total cholesterol, and triglyceride levels in serum, plasma, and heart tissue.
- Promotion of blood vessel regeneration and reduction of blood glucose levels.
- Effective reduction in the thickness of the aortic wall.
Edwards et al. [82] reported that patients with stage 1 hypertension who received 730 mg of quercetin daily for 28 days experienced significant reductions in systolic, diastolic, and mean arterial pressure. Quercetin also demonstrates notable effects in inhibiting LDL oxidation and improving endothelium-dependent vasodilation [83], while reducing adhesion molecules and other inflammatory markers.
In a study involving 93 overweight or obese subjects with a high risk of metabolic syndrome, a daily dose of 150 mg of quercetin for six weeks significantly reduced plasma concentrations of oxidized LDL, systolic blood pressure, and atherosclerosis markers [84].
Quercetin’s protective effects also involve modulation of nitric oxide (NO) levels, improvement of endothelial function, prevention of oxidative and inflammatory damage to neurons, and antiplatelet aggregation effects. Wei et al. [85] demonstrated that quercetin treatment reduced lipopolysaccharide (LPS)-induced cardiac abnormalities in mice, suggesting potential therapeutic applications for heart diseases.
Antiobesity Effects: Obesity is defined as the unhealthy expansion and accumulation of adipose tissue, which stores excess energy intake and impairs both physical and psychosocial health. It is closely associated with metabolic syndromes such as type 2 diabetes, insulin resistance, and cardiovascular diseases [86].
Leptin, a satiety and anti-obesity hormone secreted by adipocytes in response to insulin, prevents overfeeding by inhibiting AMP-activated protein kinase (AMPK) in the hypothalamus, thereby suppressing appetite. However, in obese individuals, leptin resistance occurs, leading to decreased levels of SOCS3 protein and a reduced AMP/ATP ratio, which ultimately diminishes AMPK activation.
Various strategies for obesity management include the use of bioactive polyphenols. These compounds can reduce energy and food intake, inhibit lipogenesis, and suppress preadipocyte differentiation and proliferation. Additionally, they promote energy expenditure by stimulating lipolysis and β-oxidation [87]. For example, 3T3-L1 preadipocytes have been linked to obesity-induced inflammation in both zebrafish and mouse models [88].
Quercetin has been shown to exert a direct and rapid downregulatory effect on sterol regulatory element-binding proteins (SREBP-1 and SREBP-2), which are key transcription factors regulating de novo fatty acid and cholesterol synthesis. Furthermore, quercetin reduces the activity of carbohydrate response element-binding protein (ChREBP), which is involved in regulating lipogenic genes [89].
Antidiabetic Effects: Diabetes mellitus is a chronic metabolic disorder characterized by abnormalities in carbohydrate, protein, and lipid metabolism, along with persistent hyperglycemia associated with oxidative stress. It is one of the top five leading causes of death worldwide [86].
Dysregulation of AMP-activated protein kinase (AMPK), a key regulator of various metabolic and physiological processes, contributes to chronic diseases such as obesity, inflammation, diabetes, and cancer. In diabetes treatment, AMPK activation is often targeted by synthetic drugs. In addition to pharmaceuticals, numerous natural phytochemicals, particularly polyphenols, have been shown to activate AMPK. Quercetin, a prominent polyphenol, has been demonstrated to activate AMPK and alleviate complications associated with Type 2 diabetes [90].
Polyphenolic compounds may exert antidiabetic effects by inhibiting amylin aggregation and modulating oxidative stress and inflammation, thereby promoting β-cell survival and enhancing whole-body insulin sensitivity. These compounds inhibit and destabilize amylin self-assembly by binding to folding monomers or oligomers through aromatic molecular structures combined with adjacent hydroxyl groups on single phenyl rings [91].
Recent in vitro and in vivo studies have demonstrated quercetin’s antidiabetic potential by maintaining whole-body glucose homeostasis. Quercetin inhibits glucose absorption, stimulates insulin secretion, and sensitizes insulin activity in the gut through interactions with molecular targets in the small intestine, pancreas, skeletal muscle, adipose tissue, and liver, improving glucose utilization in peripheral tissues [92].
Consumption of both low and high doses of quercetin improved hyperglycemia and hypertriglyceridemia, and enhanced antioxidant status by reducing thiobarbituric acid reactive substances (TBARS) and increasing antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) in the liver [93].
Moreover, both curcumin and quercetin modulate lysosomal enzymes (N-acetyl-β-D-glucosaminidase, β-D-glucuronidase, β-D-galactosidase, and acid phosphatase) in various tissues of streptozotocin-induced diabetic rats [94]. Due to curcumin’s low bioavailability, combining it with quercetin enhances therapeutic efficacy significantly compared to curcumin alone. Oral administration of curcumin extract combined with piperine and quercetin (100 mg/kg/day) for 28 days markedly reduced plasma glucose levels in streptozotocin- and nicotinamide-induced diabetic rats [95].
Antiviral Activity of Quercetin and COVID-19: Multiple studies have demonstrated the antiviral potential of quercetin, largely due to its inhibitory properties against various viruses. Khachatoorian et al. (2012) evaluated quercetin’s antiviral effects using an HCV cell culture system, where treatment initiated 3 hours post-infection markedly inhibited viral translation. Quercetin also showed antiviral activity against human cytomegalovirus (HCMV)-infected cells; at a concentration of 4.8 μM, it partially inhibited Immediate Early Protein production and strongly suppressed Early Protein production [96].
Both quercetin and its glycoside quercitrin exhibited potent antiviral activities against varicella-zoster virus (VZV) and HCMV by strongly suppressing the expression of lytic immediate-early genes (IEGs) [97].
In vitro studies have also revealed the anti-influenza efficacy of quercetin and its derivatives. For example, when cells were inoculated with influenza virus at a multiplicity of infection (MOI) of 0.05 or 5 and incubated with quercetin, significant inhibition of viral replication was observed over time. Notably, quercetin derivatives such as quercetin-7-O-glucoside (Q7G) demonstrated strong inhibitory activity by blocking viral RNA [98].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, has led to severe pneumonia, extreme inflammatory reactions, acute lung injury, and multiple organ dysfunction syndrome, which are primarily responsible for the disease severity. As an anti-inflammatory agent, quercetin may be effective in treating severe inflammation—a key life-threatening condition in COVID-19—by suppressing the production of pro-IL-1β and modulating the NLR family pyrin domain-containing 3 (NLRP3) inflammasome through its regulators such as TXNIP, SIRT1, and Nrf2 [99].
Available evidence also suggests that synergistic therapy combining quercetin with vitamin C exhibits antiviral and immunomodulatory properties, which may aid in the prevention and treatment of COVID-19 [100].
RESULT
Quercetin is a significant flavonoid found abundantly in many commonly consumed foods, renowned for its potent and versatile biological activities. Extensive research has demonstrated that quercetin possesses a broad spectrum of pharmacological effects, including antioxidant, antimicrobial, antidiabetic, antibacterial, anti-inflammatory, anticancer, anti-Alzheimer’s, antihypertensive, anti-allergic, anti-obesity, and antiviral properties. Thanks to these multifaceted benefits, quercetin is gaining increasing attention as a natural and cost-effective therapeutic alternative in the health field.
Its effectiveness against numerous chronic and degenerative diseases has positioned quercetin as a promising molecule in complementary and alternative medicine. However, significant gaps remain regarding its bioavailability, pharmacokinetics, and interactions with other drugs, which must be addressed to optimize its clinical application. Advanced and multidisciplinary studies are therefore critical to maximize quercetin’s therapeutic potential and ensure its safe use.
In conclusion, quercetin stands out as a strong candidate to become one of the most important natural therapeutic agents of the future, due to its scientific reliability, affordability, and broad pharmacological efficacy. To fully realize this potential, however, it is essential to deepen the scientific evidence through comprehensive research supported by clinical trials and collaborative interdisciplinary efforts. Harnessing the healing power of nature, quercetin could play a transformative role in the future of modern medicine.
ACKNOWLEDGEMENTS
This study was prepared by my student Gizem ÇİĞERCİOĞLU from Agri İbrahim Cecen University Faculty of Pharmacy research project thesis.
Conflict of interest: None
Financial support: None
Ethhas been reported to be statement: None
REFERENCE
- Grewal, A. K., Singh, T. G., Sharma, D., Sharma, V., Singh, M., Rahman, M. H., … & Abdel-Daim, M. M. (2021). Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomedicine & Pharmacotherapy, 140, 111729.
- Deepika, & Maurya, P. K. (2022). Health benefits of quercetin in age-related diseases. Molecules, 27(8), 2498.
- Batiha, G.E.; Beshbhas been reported to behy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.; Taha, A.E.; Algammal, A.M.; Elewa, Y. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374.
- DiPetrillo, A., Orrù, G., Fahas been reported to be, A., & Fantini, M. C. (2022). Quercetinanditsderivates as antiviralpotentials: A comprehensivereview. PhytotherapyResearch, 36(1), 266-278.
- TAŞCI, B.,& İlkay, K. O. C. A. (2019). Mor Soğanın (AlliumCepa L.) Önemli Bileşiği: Kersetin Ve Sağlık Üzerine Etkileri. Samsun Sağlık Bilimleri Derghas been reported to bei, 4(2), 32-37
- Londhe, V. P., Gavasane, A. T., Nipate, S. S., Bandawane, D. D., & Chaudhari, P. D. (2011). Role of garlic (Allium sativum) in various dhas been reported to beeases: An overview. Angiogeneshas been reported to be, 12(13), 129-134.
- İnanır, C., Albayrak, S. & Ekici, L. (2019). Karabuğdayın Fitokimyası, Farmakolojhas been reported to bei ve Biyofonksiyonel Özellikleri. Avrupa Bilim ve Teknoloji Derghas been reported to bei, (16), 713-722.
- Bai, C. Z., Feng, M. L., Hao, X. L., Zhong, Q. M., Tong, L. G. ve Wang, Z. H. (2015). Rutin , quercetin , andfree amino acidanalyshas been reported to be in buckwheat ( Fagopyrum ) seeds from different locations. Genetics andMolecularResearch, 14(4), 19040–19048.
- Güleç, K. (2016). Kuersetin’insiklodekstrinnanopartiküllerinin hazırlanması ve in vitro değerlendirilmesi (Master’stheshas been reported to be, Anadolu Üniversitesi).
- YAlçIn, A. S., YIlMAz, A. M., AltunDAğ, E. M., & KOçtürK, S. (2017). Kurkumin, kuersetin ve çay kateşinlerinin anti-kanser etkileri. Marmara Pharmaceutical Journal, 21(1), 19-29.
- [11]R. T. Magar and J. K. Sohng, “A review on structure, modifications and structure-activity relation of quercetin and its derivatives,” Journal of Microbiology and Biotechnology, vol. 30, no. 1, pp. 11–20, 2020.
- Li, Y. et al. Quercetin, inflammation and immunity. Nutrients 8(3), 167 (2016).
- Crespy, V., Morand, C., Manach, C., Besson, C., Demigne, C., & Remesy, C. (1999). Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. American Journal of Physiology-Gastrointestinal and Liver Physiology, 277(1), G120-G126.
- Scholz. (2007). Interactions affecting the bioavailability of dietary polyphenols in vivo. International journal for vitamin and nutrition research, 77(3), 224-235.
- Ader, P., Wessmann, A., & Wolffram, S. (2000). Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radical Biology and Medicine, 28(7), 1056-1067.
- Guo, Y., Mah, E., Davis, C. G., Jalili, T., Ferruzzi, M. G., Chun, O. K., & Bruno, R. S. (2013). Dietary fat increases quercetin bioavailability in overweight adults. Molecular nutrition & food research, 57(5), 896-905.
- de Boer, V. C., Dihal, A. A., van der Woude, H., Arts, I. C., Wolffram, S., Alink, G. M., … & Hollman, P. C. (2005). Tissue distribution of quercetin in rats and pigs. The Journal of nutrition, 135(7), 1718-1725.
- Kim, D. H., Kim, S. Y., Park, S. Y., & Han, M. J. (1999). Metabolism of quercitrin by human intestinal bacteria and its relation to some biological activities. Biological and Pharmaceutical Bulletin, 22(7), 749-751.
- Manach, C., Texier, O., Morand, C., Crespy, V., Régérat, F., Demigné, C., & Rémésy, C. (1999). Comparison of the bioavailability of quercetin and catechin in rats. Free Radical Biology and Medicine, 27(11-12), 1259-1266.
- Oliveira, E. J., & Watson, D. G. (2000). In vitro glucuronidation of kaempferol and quercetin by human UGT-1A9 microsomes. FEBS letters, 471(1), 1-6.
- Koli, R., Erlund, I., Jula, A., Marniemi, J., Mattila, P., & Alfthan, G. (2010). Bioavailability of various polyphenols from a diet containing moderate amounts of berries. Journal of agricultural and food chemistry, 58(7), 3927-3932.
- Morand C., Crespy V., Manach C., Besson C., Demigné C., Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. Amerimay Journal of Physiology 1998; 275: 212–219
- Boulton, D. W., Walle, U. K., Walle, T. (1998) Extensive binding of the bioflavonoid quercetin to human plasma proteins. J. Pharm. Pharmacol. 50: 243–249.
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205.
- Graefe, E. U., Derendorf, H., & Veit, M. (1999). Pharmacokinetics and bioavailability of the flavonol quercetin in humans. International journal of clinical pharmacology and therapeutics, 37, 219-233.
- Manach, C., Mazur, A., & Scalbert, A. (2005). Polyphenols and prevention of cardiovascular diseases. Current opinion in lipidology, 16(1), 77-84.
- Konrad, M., & Nieman, D. C. (2015). Evaluation of quercetin as a countermeasure to exercise-induced physiological stress. SPORT NUTRITION, 155.
- Moon, Y. J., Wang, L., DiCenzo, R., & Morris, M. E. (2008). Quercetin pharmacokinetics in humans. Biopharmaceutics & drug disposition, 29(4), 205-217.
- Walle, T., Walle, U. K., & Halushka, P. V. (2001). Carbon dioxide is the major metabolite of quercetin in humans. The Journal of nutrition, 131(10), 2648-2652.
- Nguyen, T. L. A., & Bhattacharya, D. (2022). Antimicrobial activity of quercetin: an approach to its mechanistic principle. Molecules, 27(8), 2494.
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetinand rutin as potentialagentsantifungalagainstCryptococcusspp etin and rutin as potentialagentsantifungalagainstCryptococcusspp. Braz. J. Biol. 2016, 76, 1029–1034. [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostaticeffect of quercetin as an antibioticalternative in vivoanditsantibacterialmechanhas been reported to bem in vitro. J. FoodProt. 2018, 81, 68–78.
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of PhosphorylatedandModifiedFlavonoids. ACS Omega 2019, 4, 12865–12871.
- Montone, A. M. I., Papaianni, M., Malvano, F., Capuano, F., Capparelli, R., & Albanese, D. (2021). Lactoferrin, quercetin, and hydroxyapatite act synergistically against Pseudomonas fluorescens. International Journal of Molecular Sciences, 22(17), 9247.
- Yin, J.; Peng, X.; Lin, J.; Zhang, Y.; Zhang, J.; Gao, H.; Tian, X.; Zhang, R.; Zhao, G. Quercetin ameliorates Aspergillus fumigatuskeratithas been reported to beby inhibiting fungalgrowth, toll-likereceptorsandinflammatorycytokines. Int. Immunopharmacol. 2021, 93, 107435.[CrossRef] [PubMed]
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplementandantimicrobialactivities of flavonoidsfromEntadaphaseoloides. Nat. Prod. Commun. 2012, 7, 867–871. [CrossRef]
- Crebelli, R., Aquilina, G., Falcone, E., & Carere, A. (1987). Urinary and faecal mutagenicity in Sprague-Dawley rats dosed with the food mutagens quercetin and rutin. Food and chemical toxicology, 25(1), 9-15.
- Chien, S. Y., Wu, Y. C., Chung, J. G., Yang, J. S., Lu, H. F., Tsou, M. F., … & Chen, D. R. (2009). Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Human & experimental toxicology, 28(8), 493-503.
- Çiftçi, R., and A. Yüce. “Effect of quercetin on homocysteine level and coronary vascular damage in rats with liver fibrosis.” (2013): 159-167.
- Elik, M., Serdaroğlu, G., & Özkan, R. (2007). Mİrisetin ve kuersetin bileşiklerinin antioksidan etkinliklerinin dft yöntemiyle incelenmesi. Fen Bilimleri Dergisi, 28(2), 53-65.
- Zu, G., Sun, K., Li, L., Zu, X., Han, T., & Huang, H. (2021). Mechanism of quercetin therapeutic targets for Alzheimer disease and type 2 diabetes mellitus. Scientific reports, 11(1), 22959.
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biologicalimportance of quercetin: A bioactiveflavonoid. Pharmacogn. Rev. 2016, 10, 84.
- Khan, M. T. H., Orhan, I., Şenol, F. S., Kartal, M. U. R. A. T., Şener, B., Dvorská, M., … & Šlapetová, T. (2009). Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chemico-Biological Interactions, 181(3), 383-389.
- Sabogal-Guáqueta, A. M., Muñoz-Manco, J. I., Ramírez-Pineda, J. R., Lamprea-Rodriguez, M., Osorio, E., & Cardona-Gómez, G. P. (2015). The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology, 93, 134-145.
- Wang, D. M., Li, S. Q., Wu, W. L., Zhu, X. Y., Wang, Y., & Yuan, H. Y. (2014). Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochemical research, 39, 1533-1543.
- Khan, H., Ullah, H., Aschner, M., Cheang, W. S., & Akkol, E. K. (2019). Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules, 10(1), 59.
- Hollman, P. C., Geelen, A., & Kromhout, D. (2010). Dietary flavonol intake may lower stroke risk in men and women. The Journal of nutrition, 140(3), 600-604.
- Perez-Vizcaino, F., & Duarte, J. (2010). Flavonols and cardiovascular disease. Molecular aspects of medicine, 31(6), 478-494.
- Rivera, L., Morón, R., Sánchez, M., Zarzuelo, A., & Galisteo, M. (2008). Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity, 16(9), 2081-2087.
- Edwards, R. L., Lyon, T., Litwin, S. E., Rabovsky, A., Symons, J. D., & Jalili, T. (2007). Quercetin reduces blood pressure in hypertensive subjects1. The Journal of nutrition, 137(11), 2405-2411.
- Egert, S., Boesch-Saadatmandi, C., Wolffram, S., Rimbach, G., & Müller, M. J. (2010). Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. The Journal of nutrition, 140(2), 278-284.
- Hertog, M. G., Feskens, E. J., Hollman, P. C., Katan, M. B., & Kromhout, D. (1994). Dietary flavonoids and cancer risk in the Zutphen Elderly Study.
- Kyle, J. A., Sharp, L., Little, J., Duthie, G. G., & McNeill, G. (2010). Dietary flavonoid intake and colorectal cancer: a case–control study. British journal of nutrition, 103(3), 429-436.
- Zhang, X., Tang, Y., Lu, G., & Gu, J. (2023). Pharmacological activity of flavonoid quercetin and its therapeutic potential in testicular injury. Nutrients, 15(9), 2231.
- Chirumbolo, S. (2010). The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation & allergy-drug targets (formerly current drug targets-inflammation & allergy)(discontinued), 9(4), 263-285.
- J. Mlcek, J., Jurikova, T., Skrovankova, S., & Sochor, J. (2016). Quercetin and its anti-allergic immune response. Molecules, 21(5), 623.
- Juríková, T., Mlček, J., Sochor, J., & Hegedűsová, A. (2015). Polyphenols and their mechanism of action in allergic immune ResponseImmune response. Global Journal of Allergy, 1(2), 037-039.
- C. Boesch-Saadatmandi, C., Loboda, A., Wagner, A. E., Stachurska, A., Jozkowicz, A., Dulak, J., … & Rimbach, G. (2011). Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. The Journal of nutritional biochemistry, 22(3), 293-299.
- Bureau, G., Longpré, F., & Martinoli, M. G. (2008). Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. Journal of neuroscience research, 86(2), 403-410.
- Nieman, D. C., Henson, D. A., Maxwell, K. R., Williams, A. S., McAnulty, S. R., Jin, F., … & Lines, T. C. (2009). Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Medicine & Science in Sports & Exercise, 41(7), 1467-1475.
- Jantan, I., Ahmad, W., & Bukhari, S. N. A. (2015). Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Frontiers in plant science, 6, 655.
- Y. Hanasaki, S. Ogawa, and S. Fukui, “The correlation between active oxygens scavenging and antioxidative effects of flavonoids,” Free Radical Biology & Medicine, vol. 16, no. 6, pp. 845–850, 1994.
- W. Y. Oh, P. Ambigaipalan, and F. Shahidi, “Preparation of quercetin esters and their antioxidant activity,” Journal of Agricultural and Food Chemhas been reported to betry, vol. 67, no. 38, pp. 10653–10659, 2019.
- Manca, M. L., Castangia, I., Caddeo, C., Pando, D., Escribano, E., Valenti, D., … & Manconi, M. (2014). Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids and Surfaces B: Biointerfaces, 123, 566-574.
- Tang, Y., Li, Y., Yu, H., Gao, C., Liu, L., Xing, M., … & Yao, P. (2014). Quercetin attenuates chronic ethanol hepatotoxicity: Implication of “free” iron uptake and release. Food and Chemical Toxicology, 67, 131-138.131–138, 2014.
- I. V. Babenkova, A. N. Osipov, and Y. O. Teselkin, “The effect of dihydroquercetin on catalytic activity of iron (II) ions in the fenton reaction,” Bulletin of Experimental Biology and Medicine, vol. 165, no. 3, pp. 347–350, 2018.
- Lim, B. O., Yu, B. P., Cho, S. I., Her, E., & Park, D. K. (1998). The inhibition by quercetin and ganhuangenin on oxidatively modified low density lipoprotein. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 12(5), 340-345.
- M. Mbikay, M., Sirois, F., Simoes, S., Mayne, J., & Chrétien, M. (2014). Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS open bio, 4, 755-762.
- Regitz, C.; Dußling, L.M.; Wenzel, U. Amyloid-beta (Aβ1–42)-induced paralyshas been reported to be in Caenorhabdithas been reported to be elegans has been reported to be inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940.
- TK Khan, T. K., Nelson, T. J., Verma, V. A., Wender, P. A., & Alkon, D. L. (2009). A cellular model of Alzheimer’s disease therapeutic efficacy: PKC activation reverses Aβ-induced biomarker abnormality on cultured fibroblasts. Neurobiology of disease, 34(2), 332-339.
- CA Dal Belo, C. A., Lucho, A. P. D. B., Vinadé, L., Rocha, L., Seibert França, H., Marangoni, S., & Rodrigues-Simioni, L. (2013). In vitro antiophidian mechanisms of Hypericum brasiliense choisy standardized extract: quercetin‐dependent neuroprotection. BioMed Research International, 2013(1), 943520.
- S. West, S., & Bhugra, P. (2015). Emerging drug targets for Aβ and tau in Alzheimer’s disease: a systematic review. British journal of clinical pharmacology, 80(2), 221-234.
- M. Sastre, M., Klockgether, T., & Heneka, M. T. (2006). Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. International Journal of Developmental Neuroscience, 24(2-3), 167-176.
- N. Sriraksa, N., Wattanathorn, J., Muchimapura, S., Tiamkao, S., Brown, K., & Chaisiwamongkol, K. (2012). Cognitive‐Enhancing Effect of Quercetin in a Rat Model of Parkinson′ s Disease Induced by 6‐Hydroxydopamine. Evidence‐Based Complementary and Alternative Medicine, 2012(1), 823206.
- Shim, J. S., Kim, H. G., Ju, M. S., Choi, J. G., Jeong, S. Y., & Oh, M. S. (2009). Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson’s disease. Journal of Ethnopharmacology, 126(2), 361-365.
- [76]Korczyn AD (2001) Hallucinations in Parkinson’s dhas been reported to beease. Lancet 358: 1031–1032.
- Haleagrahara, N., Siew, C. J., & Ponnusamy, K. (2013). Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. The Journal of toxicological sciences, 38(1), 25-33.
- Karuppagounder, S. S., Madathil, S. K., Pandey, M., Haobam, R., Rajamma, U., & Mohanakumar, K. P. (2013). Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience, 236, 136-148.
- Zhang, Z. J., Cheang, L. C. V., Wang, M. W., & Lee, S. M. Y. (2011). Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. International journal of molecular medicine, 27(2), 195-203.
- R. Sandhir, R., & Mehrotra, A. (2013). Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington’s disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1832(3), 421-430.
- J. Terao, “Factorsmodulatingbioavailability of quercetin related flavonoid sandı the consequences of their vascular function,” BiochemicalPharmacology, vol. 139, pp. 15–23, 2017.
- R. L. Edwards, T. Lyon, S. E. Litwin, A. Rabovsky, J. D. Symons, and T. Jalili, “Quercetinreducesbloodpressure in hypertensivesubjects,” TheJournal of Nutrition, vol. 137, no. 11, pp. 2405–2411, 2007.
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomhas been reported to beed double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277.
- Egert, S., Bosy-Westphal, A., Seiberl, J., Kürbitz, C., Settler, U., Plachta-Danielzik, S., … & Müller, M. J. (2009). Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. British journal of nutrition, 102(7), 1065-1074.
- Wei, X., Meng, X., Yuan, Y., Shen, F., Li, C., & Yang, J. (2018). Quercetin exerts cardiovascular protective effects in LPS-induced dysfunction in vivo by regulating inflammatory cytokine expression, NF-κB phosphorylation, and caspase activity. Molecular and cellular biochemistry, 446, 43-52.
- Hasan, A. A., Tatarskiy, V., & Kalinina, E. (2022). Synthetic pathways and the therapeutic potential of quercetin and curcumin. International Journal of Molecular Sciences, 23(22), 14413.
- Carrasco-Pozo, C., Cires, M. J., & Gotteland, M. (2019). Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. Journal of medicinal food, 22(8), 753-770.
- Seo, M. J., Lee, Y. J., Hwang, J. H., Kim, K. J., & Lee, B. Y. (2015). The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. The Journal of nutritional biochemistry, 26(11), 1308-1316.
- Damiano, F., Giannotti, L., Gnoni, G. V., Siculella, L., & Gnoni, A. (2019). Quercetin inhibition of SREBPs and ChREBP expression results in reduced cholesterol and fatty acid synthesis in C6 glioma cells. The international journal of biochemistry & cell biology, 117, 105618.
- Kismiroğlu, C., Cengiz, S., & Yaman, M. (2020). AMPK’nin Biyokimyası: Etki mekanizmaları ve diyabetin tedavisindeki önemi. Avrupa Bilim ve Teknoloji Dergisi, (18), 162-170.
- [91] Nie, T., & Cooper, G. J. (2021). Mechanisms underlying the antidiabetic activities of polyphenolic compounds: A review. Frontiers in Pharmacology, 12, 798329.
- M Eid, H., & S Haddad, P. (2017). The antidiabetic potential of quercetin: underlying mechanisms. Current medicinal chemistry, 24(4), 355-364.
- Jeong, S. M., Kang, M. J., Choi, H. N., Kim, J. H., & Kim, J. I. (2012). Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutrition research and practice, 6(3), 201-207.
- M. B. Chougala, J. J. Bhaskar, M. G. Rajan, and P. V. Salimath, “Effect of curcumin and quercetin on lysosomal enzyme activ-ities in streptozotocin-induced diabetic rats,” Clinical Nutri-tion, vol. 31, no. 5, pp. 749–755, 2012.
- Kaur, G., Invally, M., & Chintamaneni, M. (2016). Influence of piperine and quercetin on antidiabetic potential of curcumin. Journal of Complementary and Integrative Medicine, 13(3), 247-255.
- Cotin, S., Callhas been reported to bete, C. A., Mazeron, M. C., Jo, S., Kim, S., Shin, D. H., & Kim, M. S. (2020). Inhibition of SARS-CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemhas been reported to betry, 35, 145–151.
- Kim, C. H., Kim, J. E., & Song, Y. J. (2020). Antiviral activities of quercetin and has been reported to beoquercitrin against human herpesviruses. Molecules, 25(10), 2379.
- Kim, Y., Narayanan, S., & Chang, K. O. (2010). Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral research, 88(2), 227-235.
- A. Saeedi-Boroujeni and M. R. Mahmoudian-Sani, “Anti-inflammatory potential of Quercetin in COVID-19 treatment,” Journal of Inflammation, vol. 18, no. 1, p. 3, 2021.
- R. M. L. Colunga Biancatelli, M. Berrill, J. D. Catravas, and P. E. Marik, “Quercetin and vitamin C: an experimental, synerghas been reported to betic therapy for the prevention and treatment of SARS-CoV-2 related dhas been reported to beease (COVID-19),” Frontiers in Immunology, vol. 11, p. 1451, 2020.



