Ağrı Ibrahim Cecen University Faculty of Pharmacy 04100 Ağrı, Türkiye
Corresponding author email: nauzun@agri.edu.tr
Article Publishing History
Received: 14/06/2025
Accepted After Revision: 25/07/2025
Neurodegenerative disorders, particularly Alzheimer’s disease (AD), present significant global health challenges, with cholinesterase enzymes, namely acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), serving as critical therapeutic targets. The limitations of current synthetic pharmacological interventions, characterized by undesirable side effects and high costs, have intensified the scientific and clinical pursuit of novel enzyme inhibitors derived from natural products. This investigation aimed to systematically evaluate the inhibitory effects of extracts obtained from Lycopodium clavatum spores on both AChE and BChE enzymatic activities. The experimental approach involved preparing ethanol extracts from commercially acquired Lycopodium clavatum spores, followed by the assessment of enzyme activity using the standardized Ellman’s colorimetric assay. 42. The findings revealed a clear dose-dependent inhibition of both AChE and BChE by the Lycopodium clavatum spore extracts. Specifically, at a concentration of 1 mg/ml, the extract demonstrated significant inhibition of AChE (49.85 ± 1.33%, P<0.001) and BChE (71.05 ± 0.25%, P<0.01). The calculated inhibitory concentration 50% (IC50) values further indicated potency, with 1.082 μM for AChE and a notably lower 0.019 μM for BChE.
The observation that Lycopodium clavatum spores are a common and inexpensive material that allows for economic scaling is particularly noteworthy, especially in the context of potential large-scale production for various applications, including responses to bioterrorism threats. This characteristic elevates the significance of this research beyond chronic disease management, suggesting a strategic value for rapid-response public health interventions due to its affordability and widespread availability. Furthermore, the deliberate focus on inhibiting both AChE and BChE is a critical aspect of this study. While AChE is a primary therapeutic target in early AD, BChE is increasingly recognized for its compensatory role, particularly in later stages of the disease or when AChE activity declines.
An agent capable of inhibiting both enzymes could offer a more robust and comprehensive therapeutic strategy, potentially addressing a broader spectrum of cholinergic deficits and pathological progression in neurodegenerative conditions. The significantly lower IC50 for BChE (0.019 μM) compared to AChE (1.082 μM) further suggests a potent effect on BChE, which could be exploited for specific therapeutic advantages. In conclusion, Lycopodium clavatum spore extracts present as a promising natural candidate warranting further rigorous investigation for their potential therapeutic application in the development of novel treatments for neurodegenerative diseases, particularly AD.
Lycopodium Spores, Acetylcholinesterase, Butyrylcholinesterase, Neurodegenerative Diseases, Alzheimer’s Disease.
UZUN N. Investigation of Dose-Dependent Cholinesterase Inhibitory Activity of Lycopodium clavatum Spore Extracts: Implications for Neurodegenerative Disease Therapy. International Journal of Biomedical Research Science (IJBRS). 2025;01(2).
UZUN N. Investigation of Dose-Dependent Cholinesterase Inhibitory Activity of Lycopodium clavatum Spore Extracts: Implications for Neurodegenerative Disease Therapy. International Journal of Biomedical Research Science (IJBRS). 2025;01(2). Available from: <a href=”https://shorturl.at/GIhYZ“>https://shorturl.at/GIhYZ</a>
INTRODUCTİON
Lycopodium: Botanical Description and Ethnobotanical History: Lycopodium is a distinct genus of moss belonging to the family Lycopodiaceae, commonly referred to as club moss or ground pine (1). This ancient plant is characterized by its flowerless, vascular nature, existing as either terrestrial or epiphytic forms. Its morphology includes widely branched, erect stems that are densely covered with small, simple, needle-like or scale-like leaves. Each leaf possesses a single, unbranched vascular thread, a characteristic feature distinguishing it from more complex vascular plants (2). A key reproductive feature of Lycopodium is its production of a single type of spore, which is borne on the upper surface of specialized leaves known as sporophylls, arranged in a cone shape (3).
Historically, harvested Lycopodium spores, commonly known as lycopodium powder, have been utilized in various traditional practices (4). In traditional Austrian medicine, for instance, it found application as teas or compresses for a range of ailments, including disorders of the locomotor system, skin conditions, liver and bile issues, kidney and urinary tract problems, infections, rheumatism, and gout. However, it is important to note that claims regarding the efficacy of these traditional uses remain largely unproven by modern scientific standards. Beyond medicinal applications, Lycopodium has also seen non-medicinal uses, such as its controversial employment in chemical warfare testing programs by some governments (5). Its highly flammable nature also led to its use by magicians for special effects and by pharmacists in earlier times for coating pills, while homeopaths used it for respiratory conditions (6).
The juxtaposition of Lycopodium‘s historical uses—some unproven, some even misused—with its modern scientific investigation underscores a critical aspect of natural product research. The explicit mention that traditional efficacy claims are “unproven” and its historical use in “chemical warfare testing programs” highlights a tension between anecdotal or historical applications and the imperative for rigorous, evidence-based scientific assessment. This historical context provides a strong justification for the current study’s rigorous scientific approach (7).
It emphasizes the critical importance of subjecting natural products, irrespective of their traditional heritage, to modern pharmacological scrutiny to establish genuine efficacy, safety profiles, and precise mechanisms of action (8). This study, by employing controlled laboratory methods, directly addresses the gap between historical claims and validated scientific understanding, ensuring that potential therapeutic benefits are substantiated and potential toxicities, which are also noted in literature, are thoroughly understood and mitigated.
Cholinesterase Enzymes: Physiological Roles and Pathological Significance: Enzymes are fundamental biological catalysts, protein in structure, that accelerate myriad biochemical reactions essential for the metabolism of living organisms. Among these, cholinesterase enzymes play a pivotal role in neurotransmission and are of particular interest in neurodegenerative diseases (9).
Acetylcholinesterase (AChE; EC: 3.1.1.7) is a crucial member of the serine hydrolase family. Its primary physiological function is the rapid hydrolysis of the neurotransmitter acetylcholine (ACh) in the synaptic cleft, thereby terminating cholinergic signaling. Due to its central role in cognitive function, AChE has emerged as a significant therapeutic target for pharmacological interventions in neurodegenerative pathologies, most notably Alzheimer’s disease (AD) (10,11).
Butyrylcholinesterase (BChE; EC 3.1.1.8) is a non-specific cholinesterase enzyme capable of hydrolyzing a diverse range of choline-based esters. In humans, BChE is predominantly produced in the liver and circulates in blood plasma, with its synthesis encoded by the BChE gene. It shares considerable structural and functional similarity with neuronal AChE, also known as erythrocyte cholinesterase. The analysis of BChE activity in plasma is clinically valuable as a liver function test, indicating pathological processes such as hypercholinesterasemia and hypocholinesterasemia. The enzyme has an approximate half-life of 10-14 days (12).
The central cholinergic pathway is critically involved in essential cognitive functions such, as learning and memory. Neurodegenerative disorders are often characterized by severe cholinergic deficiencies, which directly contribute to profound cognitive impairment. Consequently, the inhibition of brain cholinesterases has become a cornerstone therapeutic strategy for managing cognitive decline in various neurodegenerative conditions, including AD, senile dementia, ataxia, myasthenia gravis, glaucoma, and Parkinson’s disease (13).
The description of BChE as a “nonspecific cholinesterase” that is nonetheless “very similar to neuronal acetylcholinesterase” highlights a nuanced understanding of its therapeutic significance. While AChE is the primary focus for early AD intervention, BChE activity has been observed to increase in later stages of AD, potentially compensating for declining AChE function or even contributing to pathology. Therefore, inhibiting BChE, despite its “nonspecific” label, could offer a crucial therapeutic advantage, especially in advanced disease states or when patients exhibit reduced responsiveness to selective AChE inhibitors. The inclusion of BChE as a target in this study implicitly acknowledges this evolving understanding of its role in neurodegeneration. Furthermore, its utility as a liver function test implies a systemic presence and function, necessitating careful consideration of potential systemic effects when developing BChE-targeting therapeutics (14).
The Growing Imperative for Natural Product-Derived Therapeutics: The global scientific community is engaged in an intensive search for novel and more efficacious drug candidates to address unmet medical needs (15). In this endeavor, natural products have emerged as a significant source of interest for therapeutic agents (16). This burgeoning focus on natural compounds is largely driven by the inherent limitations of conventional modern pharmaceuticals. These limitations include the frequent occurrence of undesirable side effects, the potential for increased toxic effects on patients with long-term use, and the high economic cost associated with many synthetic drugs (17).
In contrast, plant-based treatment methods offer compelling advantages, notably their affordability and typically minimal to absent side effects. This favorable profile has led to a recent resurgence in the preference for naturally sourced medicines. Consequently, modern medical science has begun to accord greater importance to these traditional and plant-derived treatments, positioning plants as a significant subject of contemporary scientific research (18).
Study Rationale and Specific Objectives: Building upon the established roles of cholinesterases in neurodegeneration and the growing interest in natural product-derived therapeutics, the current study was specifically designed to investigate the inhibitory effect of plant-derived Lycopodium spores on both acetylcholinesterase and butyrylcholinesterase enzymes. The overarching objective is to discuss the potential applicability and usability of these extracts in the therapeutic management of neurodegenerative diseases (19).
MATERIALS AND METHODS
Plant Material Acquisition and Extract Preparation: Lycopodium clavatum spores used in this investigation were acquired from a reputable commercial supplier, Sigma-Aldrich. For the preparation of extracts, the spores were meticulously dissolved in 200 ml of 60% ethanol to facilitate the extraction of bioactive compounds. The resulting crude extracts then underwent a stringent purification process. This involved filtration through 0.22 µm sterile filters (Sartorius, Germany) to remove particulate matter and microorganisms, ensuring a cleaner extract. Subsequently, the extracts were concentrated and dried using a lyophilizer, operating at a temperature of -49°C under a 3000 mT vacuum, which effectively removes solvent while preserving thermolabile compounds. Once dried, the extracts were stored at 4°C to preserve their integrity and stability until further use.
Comprehensive detailing of these experimental procedures, from extract preparation to assay conditions, is not merely a formality but a critical determinant of scientific validity. The meticulous reporting of methodology is paramount for ensuring the reproducibility of the study by independent researchers, a fundamental principle of the scientific method. Slight variations in solvent concentration (60% ethanol), filtration pore size (0.22 µm), lyophilization parameters (-49°C, 3000 mT vacuum), or storage conditions (4°C) can significantly alter the chemical profile and concentration of active compounds in natural extracts. Similarly, precise assay conditions are critical for consistent enzyme kinetics and accurate inhibition measurements. This rigor not only validates the current findings but also enables direct comparability with future studies and facilitates the eventual translation of in vitro results to vivo models, laying a robust foundation for drug development. It implicitly addresses the inherent variability of natural products, a challenge that is further discussed later in this report.
Chemicals, Enzymes, and Reagents: Commercially sourced Acetylcholinesterase (EC 3.1.1.7, Type-VI-S, derived from electric eel) and Butyrylcholinesterase (EC 3.1.1.8, derived from equine plasma) were obtained from Sigma-Aldrich for the enzymatic assays. The specific substrates used were acetylthiocholine iodide for AChE and butyrylthiocholine chloride for BChE. 5,5′-dithio-bis-nitrobenzoic acid (DTNB), a chromogenic reagent essential for colorimetric detection, was also purchased from Sigma (St. Louis, MO). All buffers and other chemical reagents utilized throughout the study were of extra pure analytical grade, ensuring high purity and minimal interference in the experimental results. Galanthamine (Reminyl® from Johnson & Johnson), a clinically approved cholinesterase inhibitor, served as the positive control for comparative analysis, providing a benchmark for the observed inhibitory activity.
The deliberate choice of Galanthamine as a standard drug serves a crucial comparative purpose. Galanthamine is an established, FDA-approved cholinesterase inhibitor widely used in the clinical treatment of mild to moderate Alzheimer’s disease. By comparing the inhibitory effects of Lycopodium extracts directly against Galanthamine, the study provides an immediate and clinically relevant benchmark for the extract’s potential therapeutic efficacy. This comparative analysis allows researchers to gauge whether the natural extract’s potency is comparable to, or significantly different from, a drug already proven effective in humans. This comparison is vital for prioritizing promising natural compounds and guiding subsequent drug development efforts, indicating whether the extract possesses sufficient in vitro activity to warrant further, more costly in vivo and clinical investigations.
Cholinesterase Inhibition Assay Protocol: The inhibitory activity of the Lycopodium extracts against cholinesterases was quantitatively determined using the well-established colorimetric method developed by Ellman et al. (1961)(20).
For the acetylcholinesterase (AChE) inhibition assay, a precise protocol was followed: 140 μl of 0.1 mM sodium phosphate buffer (pH 8.0), 20 μl of enzyme preparation, and 20 μl of the test compound solution (dissolved in methanol) were combined in each reaction vessel. This mixture was incubated at a controlled temperature for 30 minutes to allow for potential enzyme-inhibitor interactions. Following this incubation period, 10 µl of DTNB was added, and the enzymatic reaction was initiated by the addition of 10 µl of acetylthiocholine, the specific substrate for AChE.
For the assessment of butyrylcholinesterase (BChE) activity, a modification was made to the substrate: 10 µl of butyrylthiocholine chloride was substituted as the substrate, while maintaining the other components and conditions of the assay.
The hydrolysis of both acetylthiocholine and butyrylthiocholine was monitored spectrophotometrically. The formation of the yellow 5-thio-2-nitrobenzoate anion, which results from the reaction between DTNB and the thiocholines catalyzed by the enzymes, was measured at a wavelength of 412 nm. Methanol was employed as a negative control in these experiments to account for any potential solvent effects on enzyme activity.
Data Analysis and Statistical Evaluation: The percentage of enzyme inhibition was calculated based on the Michaelis–Menten model, a standard kinetic model widely used for enzyme reactions. Galanthamine, serving as the standard drug, was tested at two concentrations: 10 μg/ml and 1 mg/ml, both dissolved in methanol, to provide a comparative reference for inhibitory potency. All experimental analyses were performed in triplicate to ensure reliability and reproducibility of the results. The data obtained expressed as means, were statistically compared using the Student T-Test. A P value of less than 0.05 (P<0.05) was predefined as the threshold for statistical significance, indicating a low probability that the observed differences occurred by chance. An enzyme inhibition graph, illustrating the percentage of activity, was generated to visualize the dose-response relationship across five different concentrations of the Lycopodium spore extract.
RESULTS
The investigation into the inhibitory effects of Lycopodium clavatum spore extracts on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes yielded clear dose-dependent results, summarized in the following tables and figure.
Dose-Dependent Anticholinesterase Activity of Lycopodium clavatum Extracts
Table 1 presents the anticholinesterase activity of Lycopodium clavatum spore extracts against both AChE and BChE at two distinct concentrations.
Table 1. Anticholinesterase activity of Lycopodium clavatum spore extracts against AChE and BChE.
| % inhibition | ||||
| AChE (10 μg/ml) | BChE (10 μg/ml) | AChE (1 mg/ml) | BChE (1 mg/ml) | |
| Lycopodium clavatum | 1.56 ± 0.67 | 11.78 ± 0.31*** | 49.85 ± 1.33*** | 71.05 ± 0.25** |
Values are expressed as mean (n=6). P>0.05; * P<0.05; ** p<0.01; *** P<0.001.
At the lower concentration of 10 μg/ml, the extract exhibited minimal inhibition of AChE (1.56 ± 0.67%). In contrast, BChE inhibition at this concentration was statistically significant, reaching 11.78 ± 0.31% (P<0.001). Upon increasing the concentration to 1 mg/ml, a substantial and statistically significant inhibitory effect was observed for both enzymes: AChE inhibition reached 49.85 ± 1.33% (P<0.001), and BChE inhibition was even more pronounced at 71.05 ± 0.25% (P<0.01).
The striking difference in IC50 values between AChE and BChE is a key finding that suggests more than just general inhibition. The IC50 for BChE (0.019 μM) is considerably lower than that for AChE (1.082 μM). This differential potency is a crucial aspect for future therapeutic development, indicating that the active compounds within the
Lycopodium extract may possess a higher affinity or a more effective inhibitory mechanism specifically for BChE. If BChE inhibition is a primary therapeutic target, for instance, in later stages of AD or specific BChE-related pathologies, Lycopodium could be an exceptionally potent natural source. Conversely, if selective AChE inhibition is desired, further fractionation and isolation of specific compounds would be necessary to identify and potentially optimize the compounds responsible for AChE inhibition, or to enhance their selectivity. These finding guides future research towards either developing a potent BChE-centric therapy or pursuing selective targeting based on the desired clinical outcome.
Visual Representation of Enzyme Inhibition: Figure 1 visually illustrates the enzyme inhibition percentage as a function of increasing ethanol extract concentrations of Lycopodium spores. This graphical representation reinforces the clear dose-response relationship observed in the quantitative data, showing that higher concentrations of the extract lead to greater enzyme inhibition.
Figure 1: Enzyme inhibition percentage graph against ethanol extract concentrations of Lycopodium spores.
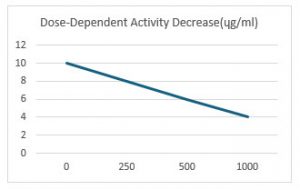
The combined data from Table 1 and Figure 1 clearly illustrates that significant inhibitory effects are observed predominantly at higher concentrations of the extract. The observation that significant AChE inhibition only occurs at higher concentration (1 mg/ml) is highly relevant for future in vivo studies and potential clinical applications. It indicates that a therapeutically effective concentration of the active compounds must be achieved. This finding directly informs dosage considerations for subsequent animal models and human trials, suggesting that simply administering small, arbitrary amounts of
Lycopodium might not yield the desired pharmacological effect. It also prompts further inquiry into the bioavailability, pharmacokinetics, and effective concentration of these compounds in vivo.
Inhibitory Concentration 50% (IC50) Values: Table 2 provides the IC50 values, which represent the concentration of the extract required to inhibit 50% of the enzyme activity, for both cholinesterase enzymes.
Table 2. Enzyme IC50 values for Lycopodium clavatum spores’ ethanol extract.
| IC50 (μM) | |
| AChE | 1.082 |
| BChE | 0.019 |
The IC50 value for AChE was determined to be 1.082 μM, while the IC50 value for BChE was notably lower at 0.019 μM. These values quantitatively confirm the extract’s potency and its differential inhibitory effect on the two enzymes.
DISCUSSION
Lycopodium Characteristics, Bioactive Constituents, and Safety Considerations: Lycopodium, commonly known as club moss, is a plant that grows along the ground and primarily reproduces by producing spores (21). Lycopodium powder is widely recognized for its use in educational demonstrations, particularly due to its highly flammable nature, which magicians historically exploited for dramatic effects (22). However, it is crucial to acknowledge the potential for moderate toxicity if the powder is inhaled or ingested. The plant is known to contain Huperzine A, a bioactive amine that is a recognized cholinesterase inhibitor (23).
Safety remains a significant concern, as studies have indicated the potential for lethality at high doses, with reported lethal doses exceeding 4 mg/kg in male rats (24). Despite the observed medical advantages of Lycopodiaceae, there is limited existing literature detailing adverse effects such as chemical pneumonia resulting from lycopodium aspiration. This gap in knowledge underscores that the comprehensive risks associated with Lycopodium use are not yet fully elucidated (25).
Contextualizing Cholinesterase Inhibition Findings in Neurodegenerative Disease: The current findings of cholinesterase inhibition by Lycopodium clavatum extracts align with previous research. Notably, alphaonocerin, another acetylcholinesterase inhibitor, was first reported to be isolated from Lycopodium clavatum by Orhan et al. (2003) (26). This reinforces the plant’s established pharmacological relevance.
The central cholinergic pathway plays a pivotal role in cognitive functions, including learning and memory. In neurodegenerative disorders, severe cholinergic deficiencies contribute significantly to cognitive impairment, making cholinesterase inhibiting a cornerstone therapeutic strategy. This approach is applied in the management of various conditions such as Alzheimer’s disease (AD), senile dementia, ataxia, myasthenia gravis, glaucoma, and Parkinson’s disease. While cholinesterase inhibitors are currently used for AD-type dementias, their utility is often constrained by associated side effects. This limitation has driven a broader discussion on the increasing appeal of natural plant products as viable therapeutic alternatives, largely due to their generally favorable side-effect profiles and greater accessibility. The potential role of various secondary metabolites, such as flavonoids, tannins, terpenoids, alkaloids, and phenols, found in natural sources in potentially mitigating disease progression is also gaining recognition (27,28).
Antioxidant Properties and Potential Synergistic Neuroprotection: Beyond cholinesterase inhibition, Lycopodium clavatum possesses inherent antioxidant properties. This is consistent with other known antioxidant-rich plants, such as Iceland moss (Cetraria islandica (L.) Ach.) and wild teasel (Dipsacus fullonum L.), whose antioxidant activities have been confirmed in various studies. Lycopodium contains key antioxidant compounds, including polyphenols, terpenoids, phenolic acids, flavonoids, alkaloids, and vitamins. Specifically, apigenin, a flavonoid polyphenol with potent antioxidant activity, has been isolated from Lycopodium clavatum (29).
The presence of a wide array of bioactive compounds and the attribution of diverse pharmacological effects (cholinesterase inhibition, antioxidant, anticancer, anti-inflammatory, etc.) to Lycopodium extracts suggest that the observed cholinesterase inhibition is likely not due to a single isolated compound but rather a collective or synergistic effect of multiple compounds present in the crude extract. This underscores the inherent complexity and potential advantage of natural product pharmacology (30). While the isolation and characterization of individual active compounds are crucial for understanding specific molecular mechanisms, the “whole extract” approach might offer synergistic benefits or a broader spectrum of action that a single isolated compound cannot replicate.
For instance, simultaneous cholinesterase inhibition and antioxidant activity, both present in Lycopodium, could provide a more comprehensive therapeutic effect against multifactorial neurodegenerative diseases where oxidative stress is a significant pathophysiological contributor (31). This suggests that future research should not only aim to isolate the primary cholinesterase inhibitors but also investigate potential synergistic interactions among the various constituents and how their combined actions contribute to the overall therapeutic potential, moving towards a multi-target therapeutic strategy.
Broader Pharmacological Activities and Chemical Diversity of Lycopodium: Beyond their cholinesterase inhibitory activity, Lycopodium spores have been observed to possess a range of other biological effects, including anticancer, immune modulatory, anti-inflammatory, and hepatoprotective properties (32). They also influence various systems, such as the reproductive and central nervous systems (33). The alkaloids found in Lycopodium are significant due to their diverse biological activities and unique chemical structures, though many of these compounds remain underexplored (34). A comprehensive analysis has identified other compounds within the plant, including vanillic, coumaric, ferulic, and syringic acids, as well as huperzine A, lycopodine, lycoflexine, Alpha-onocerin, and sporopollenin.
A critical consideration in the study of natural products is the inherent variability in active compound levels (35). The time of collection, geographical location, specific extraction procedures, and storage conditions of the plant material can significantly affect both the quantitative and qualitative profiles of their active compounds (36). This inherent variability poses a significant challenge to achieving consistent pharmacological effects. For Lycopodium extracts to be considered as legitimate therapeutic agents, robust standardization protocols for sourcing, cultivation (if applicable), extraction, and quality control are essential. Without such standardization, batch-to-batch inconsistencies in active compound profiles could lead to unpredictable potency, variable efficacy, and increased risks of adverse effects, making regulatory approval and widespread clinical adoption extremely difficult.
This highlights that while the current study demonstrates promising in vitro activity, the path to a clinically applicable drug requires substantial effort in developing rigorous standardization methods to ensure consistent therapeutic outcomes and patient safety (37). Supporting evidence from other studies, such as the reported significant reduction in tumor incidence in carcinogenically poisoned mice treated with Lycopodium clavatum spore extract, showing decreased levels of biomarkers in both liver and spleen tissues, further underscores the plant’s diverse pharmacological potential.
Comparative Analysis with Related Ethnopharmacological Studies: The findings of this study are consistent with and further supported by other ethnopharmacological investigations. Konrath et al. (2012) (38). explored the medicinal and therapeutic potentials of two Lycopodiaceae species, Lycopodium clavatum and Lycopodium thyoides, which have been traditionally used in South American folk medicine for central nervous system disorders. Their research, which evaluated alkaloid extracts for AChE and antioxidant activities, demonstrated dose- and time-dependent inhibition in various rat brain regions, including the cortex, striatum, and hippocampus, along with a reduction in lipid peroxidation. These comparative studies reinforce the notion that Lycopodium species possess multiple modes of action relevant to cognitive disorders, thereby lending scientific support to their traditional ethnobotanical uses.
The consistent referencing of Lycopodium‘s traditional uses across different cultures (Austrian, South American folk medicine) alongside its modern scientific investigation highlights a highly effective paradigm in modern drug discovery: ethnobotanical prospecting. Traditional knowledge, even if anecdotal or unproven, provides a targeted starting point for scientific inquiry, significantly narrowing the search space for novel bioactive compounds compared to random screening (39). The current study, by demonstrating the biochemical basis (cholinesterase inhibition) for some of these traditional cognitive-enhancing claims, validates this approach (40). This not only accelerates the drug discovery process but also underscores the immense value of preserving indigenous and traditional knowledge systems as a vital resource for future pharmaceutical innovations, provided they are subjected to rigorous scientific validation for safety and efficacy.
Synthesis of Findings and Future Research Directions: The investigation unequivocally demonstrates that ethanol extracts of Lycopodium clavatum spores exhibit significant dose-dependent inhibitory activity against both acetylcholinesterase and butyrylcholinesterase enzymes. This finding positions these natural extracts as promising candidates for continued research in the context of Alzheimer’s disease treatment (41).
CONCLUSION
The present study provides compelling evidence that ethanol extracts derived from Lycopodium clavatum spores possess significant dose-dependent inhibitory activity against both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. This finding underscores the compelling potential of these natural extracts as promising candidates for the development of novel therapeutic strategies aimed at combating neurodegenerative diseases, particularly Alzheimer’s disease The explicit call for “further studies” in the conclusion, following promising vitro results, highlights a critical stage in drug development.
In vitro results, while foundational, do not directly translate to clinical efficacy or safety in living organisms. This underscores the complex and multi-stage nature of pharmaceutical development. The transition from in vitro enzymatic inhibition to a demonstrable in vivo therapeutic effect requires rigorous evaluation of numerous factors that cannot be assessed in a test tube. These include the bioavailability of the active compounds (how much reaches the target site), their metabolic fate (how they are processed by the body), their ability to cross biological barriers (such as the blood-brain barrier for central nervous system effects), and their potential systemic toxicities or side effects in a complex physiological environment.
The necessity for detailed mechanistic investigations further implies the need to identify the specific compounds responsible for the observed effects and to understand their precise molecular interactions within a living system. This highlights that the current findings are a crucial first step, but significant research investment is required to bridge the gap between laboratory observation and potential clinical application.
Therefore, the critical necessity for further comprehensive in vivo studies is paramount to validate efficacy and safety in biological systems. Concurrently, detailed mechanistic investigations are required to elucidate the precise molecular interactions underlying the observed inhibition. Furthermore, systematic active compound isolation and characterization are essential to fully unlock and optimize their therapeutic potential and establish a robust safety profile for future pharmacological development.
REFERENCES
- Szypuła, W. J., & Pietrosiuk, A. (2023). Biological and ecological roles of club mosses (lycopodiaceae) alkaloids. In Plant specialized metabolites: phytochemistry, ecology and biotechnology (pp. 1-25). Cham: Springer Nature Switzerland.
- Nelson, T., & Dengler, N. (1997). Leaf vascular pattern formation. The Plant Cell, 9(7), 1121.
- Parker, S. (2009). Ferns, Mosses & Other Spore-producing Plants. Capstone.
- Wang, B., Guan, C., & Fu, Q. (2021). The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochemistry Reviews, 1-79.
- Alamgir, A. N. M., & Alamgir, A. N. M. (2017). Medicinal, non-medicinal, biopesticides, color-and dye-yielding plants; secondary metabolites and drug principles; significance of medicinal plants; use of medicinal plants in the systems of traditional and complementary and alternative medicines (CAMs). Therapeutic use of medicinal plants and their extracts: Volume 1: Pharmacognosy, 61-104.
- Dasgupta, A. (2011). Prescription Or Poison: The Benefits and Dangers of Herbal Remedies. Turner Publishing Company.
- Dubé, L., & Paré, G. (2003). Rigor in information systems positive case research: current practices, trends, and recommendations. MIS quarterly, 597-636.
- Balkrishna, A., Sharma, N., Srivastava, D., Kukreti, A., Srivastava S., & Arya, V. (2024). Exploring the safety, efficacy, and bioactivity of herbal medicines: Bridging traditional wisdom and modern science in healthcare. Future Integrative Medicine, 3(1), 35-49.
- Urlacher, V. B., Koschorreck, K., & Jaeger, K. E. (2024). 2.1 Structure of Enzymes Enzymes are biocatalysts that accelerate biochemical reactions up to 10¹7-fold and thus maintain the metabolism of all living organisms. They do so by reducing the energetic barriers that have to be overcome in the conversion of a substrate to a product. Enzymes are mainly proteins. Introduction to Enzyme Technology, 19.
- Soreq, H., Podoly, E., & Gok, M. (2020). Cholinesterases. In Encyclopedia of molecular pharmacology (pp. 1-8). Cham: Springer International Publishing.
- Arslan, S. O., Uysal, F., Koç, A., & Özünlü, S. A. Ç. Nigella sativa oil attenuates neuroinflammation and cognitive deficits in a rat model of Alzheimer’s disease. Cukurova Medical Journal, 50(2), 317-327.
- Gülçin, İ., Scozzafava, A., Supuran, C. T., Koksal, Z., Turkan, F., Çetinkaya, S., … & Alwasel, S. H. (2016). Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. Journal of enzyme inhibition and medicinal chemistry, 31(6), 1698-1702.
- Chen, Z. R., Huang, J. B., Yang, S. L., & Hong, F. F. (2022). Role of cholinergic signaling in Alzheimer’s disease. Molecules, 27(6), 1816.
- Jasiecki, J., Targońska, M., & Wasąg, B. (2021). The role of butyrylcholinesterase and iron in the regulation of cholinergic network and cognitive dysfunction in Alzheimer’s disease pathogenesis. International journal of molecular sciences, 22(4), 2033.
- Scavone, C., Di Mauro, G., Mascolo, A., Berrino, L., Rossi, F., & Capuano, A. (2019). The new paradigms in clinical research: from early access programs to the novel therapeutic approaches for unmet medical needs. Frontiers in pharmacology, 10, 111.
- Dzobo, K. (2022). The role of natural products as sources of therapeutic agents for innovative drug discovery. Comprehensive pharmacology, 408.
- David, B., Wolfender, J. L., & Dias, D. A. (2015). The pharmaceutical industry and natural products: historical status and new trends. Phytochemistry Reviews, 14, 299-315.
- Chaachouay, N., & Zidane, L. (2024). Plant-derived natural products: a source for drug discovery and development. Drugs and Drug Candidates, 3(1), 184-207.
- Potterat, O., & Hamburger, M. (2008). Drug discovery and development with plant-derived compounds. Natural Compounds as Drugs Volume I, 45-118.
- Ellman G L, Courtney K D, Andres V Jr, Feather-Stone R M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol., 7, 88-95.
- Parker, S. (2009). Ferns, Mosses & Other Spore-producing Plants. Capstone.
- Niinuma, S. A., Khudair, A. D., Habib, H., Khudair, A. D., MacKenzie, G., Atkin, S. L., & Butler, A. E. (2024). Unearthing nature’s remedy: An exploration into Lycopodium’s medicinal and therapeutic potential. Applied Materials Today, 38, 102197.
- Ferreira, A., Rodrigues, M., Fortuna, A., Falcão, A., & Alves, G. (2016). Huperzine A from Huperzia serrata: a review of its sources, chemistry, pharmacology and toxicology. Phytochemistry reviews, 15, 51-85.
- Antonelli-Ushirobira, T. M., Blainski, A., Fernandes, H. G., Moura-Costa, G. F., Costa, M. A., Campos-Shimada, L. B., … & de Mello, J. C. (2015). Acute toxicity and long-term safety evaluation of the crude extract from rhizomes of Limonium brasiliense in mice and rats. Journal of Ethnopharmacology, 174, 293-298.
- Khalid, M., Narasimhan, A. L., & Master, M. K. (2019). Chemical pneumonitis due to inhalation of lycopodium: a case report. European Journal of Medical Case Reports, 3(2), 57-60.
- Orhan I, Terzioglu S, Sener B. (2003). Alpha-onocerin: an acetylcholinesterase inhibitor from Lycopodium clavatum. Planta Med., 69(3), 265.
- Chen, Z. R., Huang, J. B., Yang, S. L., & Hong, F. F. (2022). Role of cholinergic signaling in Alzheimer’s disease. Molecules, 27(6), 1816.
- İşcan, D., Demirtaş, G., & Demirtaş, A. (2024). Relationship between nonmotor symptoms and neutrophil-to-lymphocyte ratio in Parkinson’s disease. Cukurova Medical Journal, 49(4), 916-924.
- Niinuma, S. A., Khudair, A. D., Habib, H., Khudair, A. D., MacKenzie, G., Atkin, S. L., & Butler, A. E. (2024). Unearthing nature’s remedy: An exploration into Lycopodium’s medicinal and therapeutic potential. Applied Materials Today, 38, 102197.
- Rishton, G. M. (2008). Natural products as a robust source of new drugs and drug leads: past successes and present day issues. The American journal of cardiology, 101(10), S43-S49.
- Wang, B., Guan, C., & Fu, Q. (2021). The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochemistry Reviews, 1-79.
- Wang, B., Guan, C., & Fu, Q. (2021). The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochemistry Reviews, 1-79.
- O’Connor, J. C., & Chapin, R. E. (2003). Critical evaluation of observed adverse effects of endocrine active substances on reproduction and development, the immune system, and the nervous system. Pure and applied chemistry, 75(11-12), 2099-2123.
- Wang, B., Guan, C., & Fu, Q. (2021). The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochemistry Reviews, 1-79.
- Brusotti, G., Cesari, I., Dentamaro, A., Caccialanza, G., & Massolini, G. (2014). Isolation and characterization of bioactive compounds from plant resources: the role of analysis in the ethnopharmacological approach. Journal of pharmaceutical and biomedical analysis, 87, 218-228.
- Gosslau, A., Li, S., Ho, C. T., Chen, K. Y., & Rawson, N. E. (2011). The importance of natural product characterization in studies of their anti‐inflammatory activity. Molecular Nutrition & Food Research, 55(1), 74-82.
- Ekert, J. E., Deakyne, J., Pribul-Allen, P., Terry, R., Schofield, C., Jeong, C. G., … & Rowan, W. (2020). Recommended guidelines for developing, qualifying, and implementing complex in vitro models (CIVMs) for drug discovery. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 25(10), 1174-1190.
- Konrath EL, Neves BM, Lunardi PS, Passos CS, SimoesPires A, Ortega MG et al. (2012). Investigation of the in vitro and ex vitro acetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extract. Journal of Ethnopharmacology, 139, 58-67.
- Patwardhan, B., & Chaguturu, R. (2016). Innovative approaches in drug discovery: ethnopharmacology, systems biology and holistic targeting.
- Friedman, J. I. (2004). Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology, 174, 45-53.
- Wang, B., Guan, C., & Fu, Q. (2021). The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochemistry Reviews, 1-79.



